Abstract
Background
To date, no clinical trials on the use of induction therapy before surgery have focused solely on lung squamous cell carcinoma (LSCC). We report the results of the Personalized Induction Therapy-2 (PIT-2) trial, a multicenter phase II study, performed to investigate the efficacy and safety of S-1 + cisplatin with concurrent thoracic radiotherapy (TRT) followed by surgery in patients with stage IIIA (N2) LSCC.
Methods
Patients with pathologically proven stage IIIA (N2) LSCC received induction
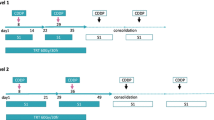
therapy comprising three cycles of S-1 + cisplatin with concurrent TRT (45 Gy in 25 fractions) followed by surgery. S-1 was administered orally at a dose of 40 mg/m2 twice daily on days 1–14, in addition to intravenous infusion of cisplatin (60 mg/m2) on day 1. The primary endpoint was 2-year progression-free survival (PFS) rate.
Results
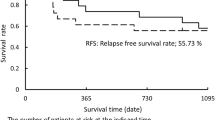
Of 45 registered patients, 43 underwent induction therapy. Of the 43 patients, 39 (91%) underwent surgery (35 lobectomies, 3 pneumonectomies, and 1 wedge resection). The 2-year PFS, 2-year overall survival, objective response rate, and pathological complete response rates were 67% (90% confidence interval [CI] 54–78%), 70% (95% CI 53–81%), 86% (95% CI 76–96%), and 39% (95% CI 23–54%), respectively. No new treatment-related adverse events occurred during the induction therapy. One case of 90-day postoperative mortality involving a patient who underwent right pneumonectomy and developed pneumonia after discharge occurred.
Conclusions
Induction therapy using S-1 + cisplatin with concurrent TRT followed by surgery is a feasible and promising treatment approach for stage IIIA (N2) LSCC.


Similar content being viewed by others
References
Koukourakis M, Skarlatos J, Kosma L, Giatromanolaki A, Yannakakis D. Radiotherapy alone for non-small cell lung carcinoma. Five-year disease-free survival and patterns of failure. Acta Oncol. 1995;34:525–30.
Martini N, Flehinger BJ, Zaman MB, Beattie EJ Jr. Results of resection in non-oat cell carcinoma of the lung with mediastinal lymph node metastases. Ann Surg. 1983;198:386–97.
Hanna N, Johnson D, Temin S, Masters G. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline Update Summary. J Oncol Pract. 2017;13:832–7.
Takamochi K, Suzuki K, Tsuboi M, et al. Randomized phase II trial of pemetrexed-cisplatin plus bevacizumab or thoracic radiotherapy followed by surgery for stage IIIA (N2) nonsquamous non-small cell lung cancer. J Thorac Cardiovasc Surg. 2022;164(3):661-671.e4.
Choy H, Akerley W, Safran H, et al. Multiinstitutional phase II trial of paclitaxel, carboplatin, and concurrent radiation therapy for locally advanced non-small-cell lung cancer. J Clin Oncol. 1998;16:3316–22.
Gandara DR, Chansky K, Albain KS, et al. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol. 2003;21:2004–10.
Vokes EE, Herndon JE 2nd, Crawford J, et al. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: cancer and leukemia group B study 9431. J Clin Oncol. 2002;20:4191–8.
Zatloukal P, Petruzelka L, Zemanova M, et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer. 2004;46:87–98.
Ichinose Y, Seto T, Sasaki T, et al. S-1 plus cisplatin with concurrent radiotherapy for locally advanced non-small cell lung cancer: a multi-institutional phase II trial (West Japan Thoracic Oncology Group 3706). J Thorac Oncol. 2011;6:2069–75.
Niho S, Yoshida T, Akimoto T, et al. Randomized phase II study of chemoradiotherapy with cisplatin + S-1 versus cisplatin + pemetrexed for locally advanced non-squamous non-small cell lung cancer: SPECTRA study. Lung Cancer. 2020;141:64–71.
Ohyanagi F, Yamamoto N, Horiike A, et al. Phase II trial of S-1 and cisplatin with concurrent radiotherapy for locally advanced non-small-cell lung cancer. Br J Cancer. 2009;101:225–31.
Sasaki T, Seto T, Yamanaka T, et al. A randomised phase II trial of S-1 plus cisplatin versus vinorelbine plus cisplatin with concurrent thoracic radiotherapy for unresectable, locally advanced non-small cell lung cancer: WJOG5008L. Br J Cancer. 2018;119:675–82.
Shimokawa T, Yamada K, Tanaka H, et al. Randomized phase II trial of S-1 plus cisplatin or docetaxel plus cisplatin with concurrent thoracic radiotherapy for inoperable stage III non-small cell lung cancer. Cancer Med. 2021;10:626–33.
Fukushima M, Sakamoto K, Sakata M, Nakagawa F, Saito H, Sakata Y. Gimeracil, a component of S-1, may enhance the antitumor activity of X-ray irradiation in human cancer xenograft models in vivo. Oncol Rep. 2010;24:1307–13.
Takagi M, Sakata K, Someya M, et al. Gimeracil sensitizes cells to radiation via inhibition of homologous recombination. Radiother Oncol. 2010;96:259–66.
Coughlin CT, Richmond RC. Biologic and clinical developments of cisplatin combined with radiation: concepts, utility, projections for new trials, and the emergence of carboplatin. Semin Oncol. 1989;16:31–43.
Yamamoto N, Yamanaka T, Ichinose Y, et al. Pooled analysis of S-1 trials in non-small cell lung cancer according to histological type. Anticancer Res. 2010;30:2985–90.
Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Japan Lung Cancer Society. General rule for clinical and pathological record of lung cancer. 7th edn. Tokyo: Kanehara & Co., Ltd; 2010.
Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–86.
Yamaguchi M, Toyokawa G, Ohba T, et al. Preoperative concurrent chemoradiotherapy of S-1/cisplatin for stage III non-small cell lung cancer. Ann Thorac Surg. 2013;96:1783–9.
Cascone T, William WN Jr, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med. 2021;27:504–14.
Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378:1976–86.
Provencio M, Nadal E, Insa A, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1413–22.
Rothschild SI, Zippelius A, Eboulet EI, et al. SAKK 16/14: durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small-cell lung cancer-a multicenter single-arm phase II trial. J Clin Oncol. 2021;39:2872–80.
Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:786–95.
Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. New Engl J Med. 2022;386:1973–85.
Acknowledgments
The authors thank the patients, their families, the JORTC Data Center, and all the investigators who participated in this study. The authors also acknowledge the following representatives of the ACTG participating institutions: Fumihiro Tanaka (University of Occupational and Environmental Health), Masanori Tsuchida (Niigata University Graduate School of Medical and Dental Sciences), Ichiro Yoshino (Chiba University Graduate School of Medicine), Satoshi Shiono (Yamagata Prefectural Central Hospital), Hiroyuki Oizumi (Yamagata University), Tomohiro Haruki (Tottori University), Norihito Okumura (Kurashiki Central Hospital), Funai Kazuhito (Hamamatsu University School of Medicine), Hiroyuki Ito (Kanagawa Cancer Center), Hirotoshi Horio (Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital), Motoki Matsuura (Hiroshima City Hiroshima Citizens Hospital), Tsuyoshi Ueno (Shikoku Cancer Center), and Tetsuzo Tagawa (Kyushu University).
Funding
This study was supported by the campus research expenses of Juntendo University. The sponsor(s) had no role in the study design, collection, analysis, and interpretation of the data, writing of the report, or decision to submit the report for publication.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Disclosures
Kazuya Takamochi, Masahiro Tsuboi, Morihito Okada, Seiji Niho, Satoshi Ishikura, Shunsuke Oyamada, Takuhiro Yamaguchi, and Kenji Suzuki have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takamochi, K., Tsuboi, M., Okada, M. et al. S-1 + Cisplatin with Concurrent Radiotherapy Followed by Surgery for Stage IIIA (N2) Lung Squamous Cell Carcinoma: Results of a Phase II Trial. Ann Surg Oncol 29, 8198–8206 (2022). https://doi.org/10.1245/s10434-022-12490-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12490-4