
Long noncoding RNA GPRC5D-AS1 in renal cell carcinoma: a molecular mechanism study
Highlight box
Key findings
• In renal cell carcinoma (RCC), long noncoding RNA (lncRNA) GPRC5D-AS1 acts as a tumor-suppressor gene.
What is known and what is new?
• lncRNA affects the occurrence and development of tumors by interacting with DNA, RNA, or proteins. lncRNA GPRC5D-AS1 can serve as a biomarker in clinical applications and the prognosis of lung squamous cell carcinoma.
• Reduced expression of lncRNA GPRC5D-AS1 activates the Wnt-β-catenin signaling pathway, promoting the occurrence and development of epithelial-mesenchymal transition. In RCC, lncRNA GPRC5D-AS1 acts as tumor-suppressor gene.
What is the implication, and what should change now?
• Our findings suggest that lncRNA GPRC5D-AS1 may serve as a potential target for RCC treatment. Further research is needed to study the role of this gene in the Wnt-β-catenin system.
Introduction
Renal cell carcinoma (RCC) is the 14th most common cancer worldwide (1), and its incidence varies according to geographical location—being higher in developed countries—and gender—being higher in males (2). RCC accounts for 2–3% of adult malignant tumors and 80–90% of adult renal malignant tumors and is second only to prostate cancer and bladder cancer among urinary system tumors; however, among these tumors, it has the highest mortality rate (3).
The occurrence and development of cancer are often closely related to the activation of oncogenes, the inactivation of tumor-suppressor genes, and abnormal changes in signaling pathways. As an intermediary of gene function, RNA regulates gene expression and biological function in tumorigenesis, and its role in these processes is garnering increased research focus (4). Among the various types of RNA, long noncoding RNA (lncRNA) is a transcription product of more than 200 nucleotides but has no obvious protein-coding potential (5,6). Despite this, lncRNA can interact with DNA, RNA, or proteins to improve basic genomic control and establish robust, flexible, and specific transcription and posttranscriptional control, thereby regulating signal transduction pathways or metabolic patterns within cells (7,8). The diversity of functions of lncRNA is closely associated with their mechanisms of action, spatiotemporal expression, and/or abundance differences (9). GPRC5D-AS1 is an RNA gene, belonging to lncRNA class, and a recent study has found that GPRC5D-AS1 can be used as a biomarker in clinical applications and the prognosis of lung squamous cell carcinoma (10). After consulting the literature, we found that lncRNA GPRC5D-AS1 has not yet been studied in relation to RCC. Therefore, we conducted this study to provide a theoretical basis for examining the mechanism of lncRNA GPRC5D-AS1 in the occurrence and development of RCC. Specifically, we observed the proliferation, invasion, and migration of RCC lines after silencing and overexpressing lncRNA GPRC5D-AS1 and measured the size of tumor volumes in nude mice. We present this article in accordance with the ARRIVE and MDAR reporting checklists (available at https://tau.amegroups.com/article/view/10.21037/tau-23-624/rc).
Methods
Materials
Renal cancer cell line 786-0 (BNCC338472) was purchased from Beina Medical Technology Co. (Wuhan, China); small interfering RNA (siRNA) and plasmid clueing (pc)DNA3.1 were purchased from Hanbio Medical Inc. (Shanghai, China); RPMI 1640 medium, fetal bovine serum (FBS), Lipofectamine 2000 reagent and TRIzol reagent kit were purchased from Thermo Fisher Scientific (Waltham, MA, USA); penicillin-streptomycin mixed solution and trypsin-EDTA (ethylenediaminetetraacetic acid) solution were purchased from Biorigin (Beijing, China); transwell cell culture inserts, 6-well plates, 12-well plates, and 24-well plates were purchased from Corning Inc. (Corning, NY, USA); Surescript First-Strand cDNA Synthesis Kit was purchased from GeneCopoeia (Rockville, MD, USA); primary antibodies for β-catenin (ab68183; AB_3073787), Ki67 (ab15580; AB_443209), proliferating cell nuclear antigen (PCNA) (ab92552; AB_10561973), N-cadherin (ab7601; AB_1310479), E-cadherin (ab212059; AB_2910596), and GAPDH (ab313650; AB_3073790), as well as secondary antibody for goat anti-rabbit immunoglobin G (IgG) (ab205718; AB_2819160), were purchased from abcam company (Cambridge, UK); and BALB/c nude mice were purchased from Sipeifu Biotechnology Co., Ltd. (Beijing, China).
Cell culture and transient transfection
After the cryopreservation tube of Renal cancer cell line 786-0 was thawed from the liquid nitrogen tank, the cells were cultured in RPMI1640 complete medium containing 10% FBS and 1% penicillin-streptomycin mixed solution and then placed in a 37 ℃, 5% CO2, saturated humidity incubator. The medium was changed every 2–3 days. When the cell density reached about 70–80%, the cells were subcultured according at a 1:3 ratio, and counting was performed after continuous culture for three generations. 786-0 cells in the logarithmic growth phase were digested with trypsin-EDTA solution and seeded in 12-well plates. After about 24 h of growth, the cell density reached about 80%. The cells were then transfected with Lipofectamine2000 reagent and divided into four groups. (I) The siGPRC5D-AS1 group was created as follows: for preparation of the siGPRC5D-AS1 diluent, 5 µL of siGPRC5D-AS1 was diluted with 120 µL Opti-MEM and mixed gently; for preparation of Lipofectamine diluent, 5 µL of Lipofectamine 2000 was diluted with 120 µL of Opti-MEM, mixed gently, and placed for 5 min at room temperature. The siGPRC5D-AS1 dilution and Lipofectamine dilution were mixed thoroughly and left at room temperature for 15 min and added to 786-0 culture wells containing serum-free medium, and then the medium containing 20% FBS was replaced after 4 h. (II) The oeGPRC5D-AS1 group was created as follows: oeGPRC5D-AS1 dilution and Lipofectamine dilution were mixed thoroughly and added to 786-0 culture wells (the methods are the same as those of the siGPRC5D-AS1 group). (III) The creation of the negative control group was as follows: for preparation of the siNC diluent, 5 µL of siRNA was diluted with 120 µL Opti-MEM and mixed gently; for preparation of the oeNC diluent, 5 µL of pcDNA3.1 was diluted with 120 µL Opti-MEM and mixed gently. The siNC dilution, oeNC dilution, and Lipofectamine dilution were mixed thoroughly and added to 786-0 culture wells (the methods are the same as those of the siGPRC5D-AS1 group). (IV) For the blank control group, no treatment was performed for the 786-0 cells. The cells of each group were collected 48 h after transfection to extract protein for subsequent use.
Quantitative real-time fluorescence polymerase chain reaction for detecting the expression of lncRNA GPRC5D-AS1 gene in each group after transfection
After transfection, the cells of each group were collected to detect the expression of lncRNA GPRC5D-AS1. According to the manufacturer’s instructions of the TRIzol, total RNA was extracted and reverse transcribed into complement DNA (cDNA) with the Surescript First-Strand cDNA Synthesis Kit The Reverse Transcription reaction system was kept at 25 ℃ for 5 min, 42 ℃ for 15 min, and 85 ℃ for 5 min, and the resulting product was stored at −20 ℃. lncRNA was amplified via quantitative real-time fluorescence polymerase chain reaction (qRT-PCR), predenatured at 95 ℃ for 3 min, and then subjected to cycling (95 ℃ for 30 s, 55 ℃ for 30 s, and 72 ℃ for 15 s), with the reaction being terminated at 72 ℃ for 5 min after 40 cycles. lncRNA GPRC5D-AS1 and internal reference gene GAPDH primers were synthesized by GeneCreate Company (Wuhan, China), as shown in Table 1.
Table 1
| Gene | Forward primer 5'-3' | Reverse primer 5'-3' |
|---|---|---|
| lncRNA GPRC5D-AS1 | GCTGTGTGAGAACTCCGTGT | ACTATCAAAGGCAGGTCGGTG |
| GAPDH | TGACAACTTTGGTATCGTGGAAGG | AGGCAGGGATGATGTTCTGGAGAG |
lncRNA, long noncoding RNA; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Cell colony formation experiment
The cells of each group in the logarithmic growth phase were added to a 12-well plate at a cell density of 600 cells per well, and the culture medium was changed every 3 days. After 2 weeks or obvious colony formation, cells were washed with phosphate-buffered saline (PBS), fixed with 4% formaldehyde, and stained with 0.1% crystal violet. Photographs were captured to calculate the colony formation rate.
Cell scratch assay
The cells of each group in the logarithmic growth phases were evenly inoculated onto a 6-well plate, and when the cell density reached 80%, the cells were scratched along the ruler by a 200 µL gun head, with each scratch being 1 cm apart. The cells were washed with PBS three times, and the serum-free medium was replaced and cultured in a 5% CO2 incubator with saturated humidity at 37 ℃. Samples were photographed at 0, 24, and 48 h.
Transwell assay
Medium (600 µL) containing 20% FBS was added to each lower chamber, and the transwell inserts were placed on top of the lower chambers. After routine digestion and centrifugation, the cells of each group were resuspended with serum-free medium, the cell density was adjusted to 1×105/mL, and 0.2 mL cell suspension was added to each upper chamber. After 24 h of cultivation, the upper chambers were removed and the liquid was sucked away. The noninvasive cells on the membrane were wiped clean with cotton swabs, and the membrane was washed twice with PBS, fixed with 4% formaldehyde, and stained with 0.1% crystal violet. The upper chambers were scrubbed clean with cotton swabs, and after air-drying, the cells on the upper chambers were photographed and counted under a microscope.
Western blotting for detecting protein expression
Each group of treated cells was collected and lysed with radioimmunoprecipitation assay (RIPA) lysis buffer to extract proteins. The protein levels of β-catenin, Ki67, PCNA, N-cadherin, and E-cadherin in each group were detected via Western blotting, with GAPDH being used as the internal reference. The protein was separated with sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to a polyvinylidene fluoride (PVDF) membrane. The PVDF membrane was incubated overnight with the first antibody and then incubated with the second antibody for 1 h. Finally, imaging was performed using developer, and the gray-scale values of each band were analyzed. Western blot was repeated three times, and the average gray-scale values were taken.
Tumor formation experiment in nude mice
A protocol was prepared before the study without registration. The animal experiments in this study were approved by Laboratory Animal Ethical and Welfare Committee of North China University of Science and Technology Affiliated Hospital (No. SQ20230206) with oversight of the facility in which the studies were conducted, in compliance with the national guidelines for the care and use of animals. Twelve 4-week-old nude mice, weighing 15–18 g, were randomly divided into four groups (n=3): the siGPRC5D-AS1 group, the oeGPRC5D-AS1 group, the negative control group, and the blank control group. Random numbers were generated using the standard = RAND() function in Microsoft Excel. An individual nude mouse was considered the experimental unit within the studies. Nude mice were kept in a dedicated laboratory space. Same sex mates were housed together in individually ventilated cages with three mice per cage. All mice were maintained on a regular diurnal lighting cycle (12-h light-dark cycle) with ad libitum access to food and water. Chopped corn cob was used as bedding. Periods of physiological or procedural acclimatization were not included in the study protocol. For each animal, two different investigators were involved. The first investigator (M.J.) administered the treatment based on the randomization table and was the only person aware of the treatment group allocation. A second investigator (X.C.) was responsible for the anesthetic and surgical procedure. The cells of each group in the logarithmic growth phase were digested and centrifuged in routine fashion, and then the cell precipitate was resuspended with PBS to ensure that the concentration of the cell suspension was 3×107/mL. Each 4-week-old nude mouse was inoculated with 0.2 mL of cell suspension into the subcutaneous area of the posterior armpit. The volume of tumor and the weight of nude mice were measured at 1 week, 2 weeks, 3 weeks, and 4 weeks after inoculation, and the differences among groups were compared. Testing order was random, with each animal tested at a different time each test. The animals were included in the study if the animal was successfully inoculated with RCC cells and were excluded if they died prematurely (preventing the collection of histological data). The animals were monitored twice daily. Health was monitored according to weight (once weekly), food and water intake, and general assessment of animal activity, panting, and fur condition. The maximum size the tumors were allowed to grow in the mice before surgery was 2,000 mm3. No adverse events occurred in nude mice during the experiment. To alleviate pain in nude mice and ensure the smooth progress of the experiment, a 0.1 mL injection of 10% chloral hydrate solution was administered intraperitoneally 15 min before the surgery.
Statistical analysis
All the data were analyzed with SPSS 26.0 statistical software (IBM Corp.), and the measurement data are expressed as the mean ± standard deviation. The Mann-Whitney test was used for comparing two groups, while one-way analysis of variance (ANOVA) was used for comparing multiple groups. A P value <0.05 indicated statistical significance.
Results
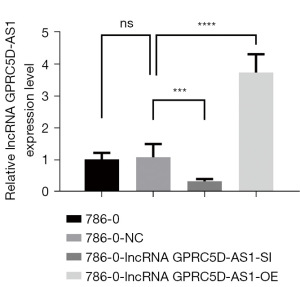
Expression of lncRNA GPRC5D-AS1 gene in 786-0 cells after transfection
Compared with that of the blank control group, the relative expression of lncRNA GPRC5D-AS1 of the negative control group was not significantly different (P>0.05); compared with that of the negative control group, the relative expression level of lncRNA GPRC5D-AS1 of the siGPRC5D-AS1 group was significantly decreased (P<0.05); compared with that of the negative control group, the relative expression level of lncRNA GPRC5D-AS1 of the oeGPRC5D-AS1 group was significantly increased (P<0.05) (Figure 1).
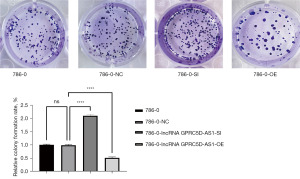
The effect of silencing and overexpressing GPRC5D-AS1 gene on the proliferation of 786-0 cells
In cell colony formation experiment, compared with that of the blank control group, the cell proliferation number in the negative control group was not significantly different (P>0.05); compared with that of the negative control group, the cell proliferation number in the siGPRC5D-AS1 group was significantly increased (P<0.05); and compared with that of the negative control group, the cell proliferation number in the oeGPRC5D-AS1 group was significantly decreased (P<0.05) (Figure 2).
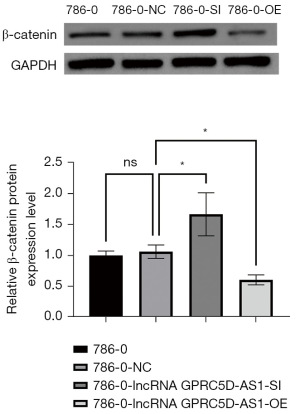
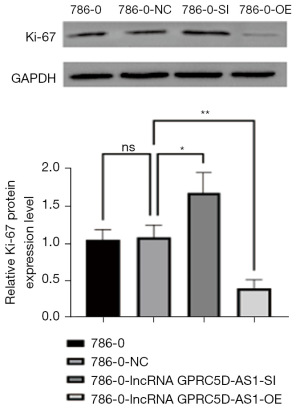
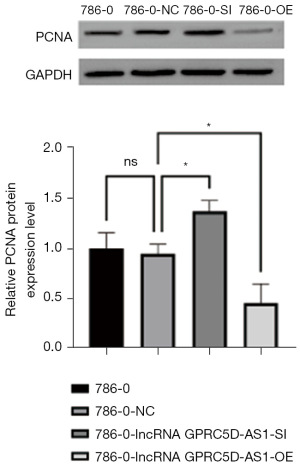
The effect of silencing and overexpressing GPRC5D-AS1 gene on the expression of proliferation-related protein in RCC
Compared with that of blank control group, the expression of β-catenin, Ki67 and PCNA protein related to proliferation in the negative control group was not significantly different (P>0.05); compared with that of the negative control group, the expression of β-catenin, Ki67 and PCNA protein in the siGPRC5D-AS1 group was significantly increased (P<0.05); compared with that of the negative control group, the expression of β-catenin, Ki67 and PCNA protein in the oeGPRC5D-AS1 group was significantly decreased (P<0.05) (Figures 3-5).
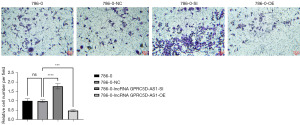
The effects of silencing and overexpressing GPRC5D-AS1 gene on the invasion and migration of 786-0 cells
In the transwell assay experiments, compared with that of the blank control group, the number of invasive cells in the negative control group was not significantly different (P>0.05); compared with that of the negative control group, the number of invasive cells in the siGPRC5D-AS1 group was significantly increased (P<0.05); and compared with that of negative control group, the number of invasive cells in the oeGPRC5D-AS1 group was significantly decreased (P<0.05) (Figure 6).
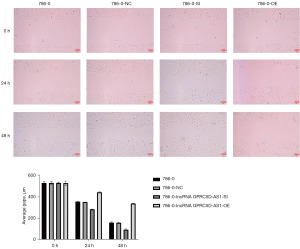
In the cell scratch test, compared with that of the blank control group, the migration distance in the negative control group was not statistically different at 24 and 48 h (P>0.05); compared with that of the negative control group, the migration distance of the siGPRC5D-AS1 group at 24 and 48 h was significantly longer (P<0.05); and compared with that of the negative control group, the oeGPRC5D-AS1 group had significantly shorter migration distances at 24 and 48 h (P<0.05) (Figure 7).
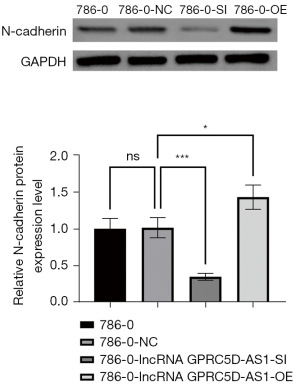
The effect of silencing and overexpressing GPRC5D-AS1 gene on the expression of invasion- and migration-related proteins in RCC
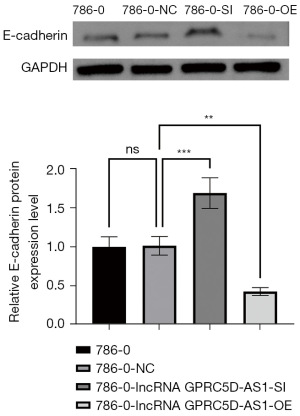
Compared with that of the blank control group, the expression of N-cadherin and E-cadherin protein related to the invasion and migration in the negative control group was not significantly different (P>0.05); compared with that of the negative control group, the expression of N-cadherin protein in the siGPRC5D-AS1 group was significantly increased (P<0.05) while the expression of E-cadherin protein was significantly decreased (P<0.05); compared with that of the negative control group, the expression of N-cadherin protein in the oeGPRC5D-AS1 group was significantly decreased (P<0.05) while the expression of E-cadherin protein was significantly increased (P<0.05) (Figures 8,9).
The effect of silencing and overexpressing GPRC5D-AS1 gene on the tumor volume and weight of nude mice
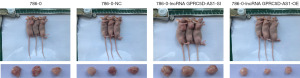
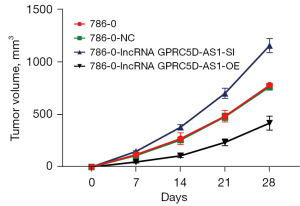
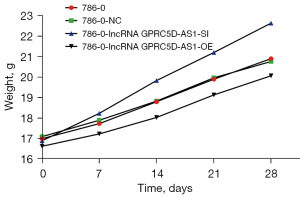
All nude mice were successfully inoculated, and there were no premature deaths among them. Detailed data on the body weight and tumor volume of nude mice can be found in the supplementary materials. Compared with that of blank control group, the tumor volume and weight of nude mice in the negative control group were not significantly different (P>0.05). However, compared with those of the negative control group, the tumor volume and weight of mice of the siGPRC5D-AS1 group showed a significant increase (P<0.05). On the other hand, compared with those of the negative control group, the tumor volume and weight of mice of the oeGPRC5D-AS1 group showed a significant decrease (P<0.05) (Figures 10-12).
Discussion
RCC is one of the more common malignant tumors in humans, with a rising morbidity and mortality (2). According to data from the Chinese Cancer Registration Annual Report, the incidence of RCC in China is increasing, and the crude death rate and standardized death rate of RCC in both men and women are on the rise (11). The most common subtype of RCC is clear-cell RCC (12), and the other subtypes are collectively referred to as non-clear cell RCC by clinical doctors. Many patients remain asymptomatic for an extended period, but when the classic triad of abdominal pain, hematuria, and abdominal mass appears, this often indicates that the disease has entered the late stage (13). The treatment for advanced or metastatic RCC primarily involves systemic drug therapy supplemented by tumor reduction surgery for the primary or metastatic sites (14). However, the prognosis is very poor, with a 5-year survival rate of only 10% to 20%, mainly attributed to the complexity and heterogeneity of the tumor microenvironment (TME). The early diagnosis and treatment of RCC are crucial, but reliable and accurate biomarkers for diagnosing RCC are lacking. In-depth research on the molecular mechanism of RCC is crucial for early diagnosis, identification of new therapeutic targets, and prognostic assessment.
RNA, as a mediator of gene function, has received increased attention due to its role in regulating gene expression and biological functions in tumor development. Noncoding RNA, such as lncRNA, circulating RNA, and microRNA (miRNA), participate in the occurrence and progression of various cancers by affecting transcription, splicing, and translation (15,16). Therefore, the changes in expression levels and functions of these RNA types in different tumors suggest their potential use as biomarkers for cancer diagnosis (17) and as potential targets for cancer therapy (18,19). The function of lncRNA is closely related to its subcellular localization. In the nucleus, lncRNA regulates gene expression at the epigenetic and transcriptional levels, while in the cytoplasm, it regulates gene expression at the posttranscriptional and translational levels. Abnormal expression of lncRNA has been shown to exhibit tumor-suppressive or oncogenic effects and plays an important role in tumor development (20). The pathogenesis of lncRNA in RCC has been extensively studied. As one of the most researched genes, lncRNA regulates the biological characteristics of cancer, such as cell proliferation, migration, invasion, and apoptosis, through the lncRNA-miRNA-messenger RNA (mRNA) axis (21). Small non-coding RNA (sncRNA) is a series of RNA molecules approximately 19 to 25 nucleotides in length. miRNA is a subclass of sncRNA that can induce gene regulation by binding to the 3' untranslated region (3'UTR) and 5' untranslated region (5'UTR) sequences of target genes (22,23). More and more research has found that miRNA has close interactions with lncRNA. The main regulatory mode is that lncRNA can act as a sponge to interact with miRNA, inhibiting the binding of miRNA to its target genes, thus leading to changes in the expression of the target genes (24). Some oncogenic miRNA are upregulated in RCC, while some tumor-suppressive miRNA are downregulated. Therefore, the binding of lncRNA with oncogenic miRNA will lead to tumor suppression. For example, the interaction between lncRNA TCL6 and miR-155 affects the Src/Akt pathway to inhibit RCC metastasis (25). The interaction of lncRNA with tumor-suppressive miRNA will promote tumor growth. For instance, lncRNA PVT1 can activate the PI3K/AKT and MAPK/ERK signaling pathways to promote RCC cell proliferation by directly targeting miR-328-3p (26).
The TME of RCC is a highly structured ecosystem, composed of various components including immune cells, non-immune cells, extracellular matrix (ECM), and signaling molecules. TME plays a crucial role in tumor formation, growth, angiogenesis, metastasis, and affects the efficacy of drug treatments. Therefore, a burgeoning area of cancer therapy is targeting the components within the TME (27,28). lncRNA LINC00973 induces the expression of Siglec-15. Upregulation of Siglec-15 can inhibit antigen-specific T cell responses, thereby enhancing tumor immune suppression (29). lncRNA CRNDE can promote tumor metastasis by activating the epithelial-mesenchymal transition (EMT) process through sponging miR-136-5p (30). Lnc ARSR (activated in RCC with sunitinib resistance) is highly expressed in extracellular vesicles derived from RCC, contributing to cytokine secretion, macrophage phagocytosis, and angiogenesis (31). LncRNA PCGEM1 upregulates FGF2 to promote RCC cell proliferation and migration (32).
lncRNA GPRC5D-AS1 is located in the human chromosome 12p13.1 region and is an antisense RNA1 of GPRC5D and HEBP1, which are closely associated with the occurrence of colorectal cancer (33). In this study, to investigate the role of lncRNA GPRC5D-AS1 in RCC, 786-0 cells were transfected with siRNA and pCDNA3.1 to silence and overexpress lncRNA GPRC5D-AS1, respectively. qRT-PCR was used to detect the expression differences of lncRNA GPRC5D-AS1 in each group after transfection, and the results indicated successful transfection. Cell and animal experiments showed that silencing lncRNA GPRC5D-AS1 enhanced the proliferation, invasion, and migration abilities of 786-0 cells, while overexpressing lncRNA GPRC5D-AS1 weakened these abilities.
The Wnt-β-catenin system is an evolutionarily conserved signaling pathway that plays a crucial role in regulating embryonic development, cell differentiation, proliferation, and adult stem cell homeostasis (34,35). It is typically inhibited during adulthood but can be reactivated during organ injury and regeneration (36). The abnormal activation of this signaling pathway is closely related to the increase of tumor incidence, malignant progression, poor prognosis, and even the increase of related mortality (37,38). The Wnt-β-catenin pathway consists of four components: extracellular signaling segment, membrane segment, cytoplasmic segment, and nuclear segment. The pathway is highly conserved: when cells are not subjected to Wnt signaling stimulation, most of the β-Catenin in the cytoplasm binds to cadherin proteins on the cell membrane, participating in cell adhesion; when the secreted ligand protein Wnt binds to the frizzled protein receptor on the membrane surface, the intracellular disheveled protein is activated and the process of complex formation via APC, axin, and GSK-3β is inhibited, leading to the dephosphorylation of β-Catenin protein to stabilize the free-state β-catenin protein in the cytoplasm. The accumulated β-catenin in the cytoplasm enters the nucleus and binds to the lymphoid enhancer factor/T cell factor (LEF/TCF) transcription factor family, initiating the transcription of downstream target genes, and its abnormal expression or activation can cause tumor development (39,40). lncRNA has a prominent regulatory role in the Wnt-β-catenin pathway: The increased expression of MSC-AS1, FAST, JPX, and LALR1 directly or indirectly enhances the stability of β-catenin, thereby activating the Wnt-β-catenin pathway (41,42); meanwhile, the decreased expression of OTUD6B-AS1 indirectly activates the Wnt-β-catenin pathway (43). Ki67 is a proliferation-related protein whose function is closely related to mitosis and is indispensable in cell proliferation. In general, the faster the tumor grows and the poorer the tissue differentiates, the higher Ki67 expression will be. Therefore, it is widely used as a proliferation marker in routine pathological studies. A study has showed the correlation of Ki-67 expression to stage and metastasis in RCC (44). PCNA is a protein located in the cell nucleus, serving as an auxiliary protein for DNA polymerase δ. It plays a crucial role in initiating cell proliferation and serves as a good indicator of the cells’ proliferative status. Therefore, in recent years, there has been a surge in research on PCNA, especially in the field of tumors, both domestically and internationally (45). In our study, Western blot results showed that silencing lncRNA GPRC5D-AS1 leads to a significant increase in β-Catenin, Ki67, and PCNA protein in RCC, suggesting that decreasing the expression of lncRNA GPRC5D-AS1 promotes the activation of the Wnt-β-catenin pathway to affect the proliferation ability of RCC.
During the process of EMT, epithelial cells lose their polarity, reduce contact with surrounding cells and stromal cells, decrease interactions between cells, and enhance cell migration and motility. At the same time, the cell changes its phenotype, losing epithelial characteristics such as keratin filaments and E-cadherin. Loss of E-cadherin expression is considered the most significant feature of EMT. The decrease of E-cadherin level can lead to reduced cell adhesion, making cells more prone to invasion and metastasis, with the interstitial phenotype, characterized by vimentin and N-cadherin expression, being increased (46,47). EMT is an important process in embryonic development and tissue regeneration. However, the abnormal reactivation of EMT is associated with the malignancy of tumor cells during cancer progression and metastasis (48). Relevant research has shown that the Wnt-β-catenin pathway integrates with the RTK-Ras-MAPK pathway and the PI3K-ILK-PKB pathway to collectively inhibit GSK-3β activity and upregulate snail levels so that the expression of E-Cadherin is decreased, thus promoting the occurrence and development of EMT and tumor metastasis (49). In our study, Western blot results showed that silencing the expression of lncRNA GPRC5D-AS1 significantly decreases E-cadherin protein levels and significantly increases N-cadherin protein levels in renal cancer cell lines, suggesting that silencing lncRNA GPRC5D-AS1 expression promotes mesenchymal transition, thereby enhancing the invasive and migratory abilities of renal cancer cells.
The abnormal levels of lncRNA in the blood of cancer patients are closely associated with the presence of tumors, and the high stability and relative abundance of circulating lncRNA may make it a more reliable cancer biomarker. For example, lncRNA GIHCG and ARSR are significantly elevated in the blood of RCC patients, and the levels of circulating GIHCG and ARSR decrease significantly after RCC tumor resection (50). In fact, the diagnostic potential of circulating lncRNA has not yet been fully realized, as single lncRNA may have poor sensitivity or specificity for specific cancer types. For instance, lncRNA MALAT-1 showed only 58.6% sensitivity when tested in plasma samples from prostate cancer patients and healthy controls. This moderate sensitivity implies that using MALAT-1 as a blood-based prostate cancer biomarker may lead to a large number of false-negative results, possibly due to some degree of degradation of MALAT-1 in the blood (51). The current common solution is to combine multiple circulating lncRNA, which provides better diagnostic performance than most individual circulating lncRNA. For example, a combination of lncRNA LET, PVT1, PANDAR, PTENP1, and linc00963 identified RCC samples with an area under the receiver operating characteristic (ROC) curve (AUC) of 0.823 (52).
Immune-related genes (IRGs) are not only associated with the response to immunotherapy, but also with the prognosis of RCC patients. Wan et al. constructed a risk model using 7 IRGs, and the risk score generated by this model can serve as an independent prognostic marker to distinguish RCC patients with different survival outcomes (53). The IRG SEMA3G is a favorable prognostic biomarker for RCC, while the lncRNA TBX2-AS1 can bind to miR-146a/b-5p to induce downregulation of SEMA3G (54). lncRNA can also serve as prognostic biomarkers for RCC. For example, lncRNA CADM1-AS1 and lncRNA PGM5-AS1 show decreased expression in RCC tissues and can act as independent prognostic factors for overall survival (OS) (55,56).
The treatment options for advanced metastatic RCC are limited, and targeted drugs and immunosuppressants are usually recommended. However, a large number of RCC patients are completely resistant to targeted therapy, leading to treatment failure and minimal clinical benefit (27). Many studies have shown that the abnormal expression of lncRNA can enhance the resistance of RCC patients to targeted drugs. For example, the high expression of lncRNA SNHG12 and lncRNA HOTAIR in RCC patients can enhance resistance to sunitinib (57,58). Therefore, altering the expression levels of lncRNA may provide new therapeutic targets for reversing resistance to targeted drugs. Antisense oligonucleotide (ASO) technology and nanoparticle-mediated RNA interference can be used to knock down overexpressed oncogenic lncRNA in cancer, and have shown promising anti-cancer effects (59). For example, targeting lncRNA WISP1-AS1 can regulate the transcription of Egr-1 and E2F genes, leading to apoptosis in RCC cells (60).
Conclusions
In summary, silencing the expression of lncRNA GPRC5D-AS1 can promote the proliferation, invasion, and migration ability of renal cancer cells, while overexpression can effectively inhibit this ability. We selected a minimum sample size because this is the first time lncRNA GPRC5D-AS1 has been studied regarding its role in RCC; therefore, the initial intention was to collect basic evidence regarding the use of this gene in more complex experimental designs. Our experimental results indicate that silencing lncRNA GPRC5D-AS1 can effectively reduce the tumor volume of RCC in nude mice. This finding may be clinically relevant to the abnormal expression of lncRNA in RCC cases, as a large number of lncRNAs have been shown to be associated with the occurrence and development of RCC. In the discussion section, the researchers listed some lncRNA that have an impact on various components of the TME of RCC and proposed some potential directions for drug development. Therefore, given the findings of this study that lncRNA GPRC5D-AS1 can inhibit the proliferation and invasion of 786-0, there is reason to believe that this lncRNA may act on certain components of the TME of RCC and exert an anti-cancer effect. Our findings may provide potential therapeutic targets for the treatment of RCC. However, there were some limitations in this study. The small number of nude mice may lead to sample selection bias. Since it is a basic experimental research, it only discusses the expression of lncRNA GPRC5D-AS1 in renal cancer cell lines and its impact on the proliferation and invasion capabilities of renal cancer cell lines. Therefore, whether lncRNA GPRC5D-AS1 can serve as a biomarker for risk stratification, diagnosis, treatment, and prognosis of RCC patients requires the collection of tissue samples from clinical patients to conduct further mechanistic studies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE and MDAR reporting checklists. Available at https://tau.amegroups.com/article/view/10.21037/tau-23-624/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-624/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-624/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-624/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments were performed under a project license (No. SQ20230206) granted by the Laboratory Animal Ethical and Welfare Committee of North China University of Science and Technology Affiliated Hospital, in compliance with the national guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Wein AJ, Kavoussi LR, Partin AW, et al. editors. Campbell-Walsh Urology: 4-Volume Set. 11th Edition. Elsevier, 2016.
- Tang Q, Li L, Wang Y, et al. RNA modifications in cancer. Br J Cancer 2023;129:204-21. [Crossref] [PubMed]
- Nojima T, Proudfoot NJ. Mechanisms of lncRNA biogenesis as revealed by nascent transcriptomics. Nat Rev Mol Cell Biol 2022;23:389-406. [Crossref] [PubMed]
- Yang M, Lu H, Liu J, et al. lncRNAfunc: a knowledgebase of lncRNA function in human cancer. Nucleic Acids Res 2022;50:D1295-306. [Crossref] [PubMed]
- Hao L, Wu W, Xu Y, et al. LncRNA-MALAT1: A Key Participant in the Occurrence and Development of Cancer. Molecules 2023;28:2126. [Crossref] [PubMed]
- Herman AB, Tsitsipatis D, Gorospe M. Integrated lncRNA function upon genomic and epigenomic regulation. Mol Cell 2022;82:2252-66. [Crossref] [PubMed]
- Grammatikakis I, Lal A. Significance of lncRNA abundance to function. Mamm Genome 2022;33:271-80. [Crossref] [PubMed]
- Ning J, Wang F, Zhu K, et al. Characterizing the Copy Number Variation of Non-Coding RNAs Reveals Potential Therapeutic Targets and Prognostic Markers of LUSC. Front Genet 2021;12:779155. [Crossref] [PubMed]
- Zheng RS, Zhang SW, Sun KX, et al. Cancer statistics in China, 2016. Zhonghua Zhong Liu Za Zhi 2023;45:212-20. [PubMed]
- Montironi R, Cimadamore A. Tumors of the Urinary System and Male Genital Organs: 2022 World Health Organization Classification and Multidisciplinarity. Eur Urol 2022;82:483-6. [Crossref] [PubMed]
- Ng KL. The Etiology of Renal Cell Carcinoma and Upper Tract Urothelial Carcinoma. In: Barber N, Ali A. editors. Urologic Cancers. Brisbane: Exon Publications, 2022.
- Benamran D, Albiges L, Bex A, et al. Treatment Options for De Novo Metastatic Clear-cell Renal Cell Carcinoma: Current Recommendations and Future Insights. Eur Urol Oncol 2022;5:125-33. [Crossref] [PubMed]
- Delaunay S, Frye M. RNA modifications regulating cell fate in cancer. Nat Cell Biol 2019;21:552-9. [Crossref] [PubMed]
- Wilkinson E, Cui YH, He YY. Roles of RNA Modifications in Diverse Cellular Functions. Front Cell Dev Biol 2022;10:828683. [Crossref] [PubMed]
- Delaunay S, Helm M, Frye M. RNA modifications in physiology and disease: towards clinical applications. Nat Rev Genet 2024;25:104-22. [Crossref] [PubMed]
- Mateo J, Porta N, Bianchini D, et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, andomized, phase 2 trial. Lancet Oncol 2020;21:162-74. [Crossref] [PubMed]
- Mehta J. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N Engl J Med 2021;384:e91. [Crossref] [PubMed]
- Xing C, Sun SG, Yue ZQ, et al. Role of lncRNA LUCAT1 in cancer. Biomed Pharmacother 2021;134:111158. [Crossref] [PubMed]
- Gao X, Zhang H, Zhang C, et al. The emerging role of long non-coding RNAs in renal cell carcinoma progression and clinical therapy via targeting metabolic regulation. Front Pharmacol 2023;14:1122065. [Crossref] [PubMed]
- Zhou P, Xu W, Peng X, et al. Large-scale screens of miRNA-mRNA interactions unveiled that the 3’UTR of a gene is targeted by multiple miRNAs. PLoS One 2013;8:e68204. [Crossref] [PubMed]
- Ørom UA, Nielsen FC, Lund AH. MicroRNA-10a binds the 5’UTR of ribosomal protein mRNAs and enhances their translation. Mol Cell 2008;30:460-71. [Crossref] [PubMed]
- Jin Y, Huang R, Xia Y, et al. Long Noncoding RNA KIF9-AS1 Regulates Transforming Growth Factor-β and Autophagy Signaling to Enhance Renal Cell Carcinoma Chemoresistance via microRNA-497-5p. DNA Cell Biol 2020;39:1096-103. [Crossref] [PubMed]
- Kulkarni P, Dasgupta P, Hashimoto Y, et al. A lncRNA TCL6-miR-155 Interaction Regulates the Src-Akt-EMT Network to Mediate Kidney Cancer Progression and Metastasis. Cancer Res 2021;81:1500-12. [Crossref] [PubMed]
- Xie G, Zheng X, Zheng Z, et al. The ceRNA PVT1 inhibits proliferation of ccRCC cells by sponging miR-328-3p to elevate FAM193B expression. Aging (Albany NY) 2021;13:21712-28. [Crossref] [PubMed]
- Zhang Q, Ren H, Ge L, et al. A review on the role of long non-coding RNA and microRNA network in clear cell renal cell carcinoma and its tumor microenvironment. Cancer Cell Int 2023;23:16. [Crossref] [PubMed]
- de Visser KE, Joyce JA. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023;41:374-403. [Crossref] [PubMed]
- Liu Y, Li X, Zhang C, et al. LINC00973 is involved in cancer immune suppression through positive regulation of Siglec-15 in clear-cell renal cell carcinoma. Cancer Sci 2020;111:3693-704. [Crossref] [PubMed]
- Zhang Y, Lan X, Wang Y, et al. CRNDE mediates the viability and epithelial-mesenchymal transition of renal cell carcinoma via miR-136-5p. J Recept Signal Transduct Res 2021;41:234-44. [Crossref] [PubMed]
- Zhang W, Zheng X, Yu Y, et al. Renal cell carcinoma-derived exosomes deliver lncARSR to induce macrophage polarization and promote tumor progression via STAT3 pathway. Int J Biol Sci 2022;18:3209-22. [Crossref] [PubMed]
- Cai X, Zhang X, Mo L, et al. LncRNA PCGEM1 promotes renal carcinoma progression by targeting miR-433-3p to regulate FGF2 expression. Cancer Biomark 2020;27:493-504. [Crossref] [PubMed]
- Jin M, Gu S, Ye D, et al. Association between genetic variants in the promoter region of a novel antisense long noncoding RNA RP11-392P7.6 and colorectal cancer risk. Environ Mol Mutagen 2017;58:434-42. [Crossref] [PubMed]
- Jung YS, Park JI. Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp Mol Med 2020;52:183-91. [Crossref] [PubMed]
- Hayat R, Manzoor M, Hussain A. Wnt signaling pathway: A comprehensive review. Cell Biol Int 2022;46:863-77. [Crossref] [PubMed]
- Schunk SJ, Floege J, Fliser D, et al. WNT-β-catenin signalling – a versatile player in kidney injury and repair. Nat Rev Nephrol 2021;17:172-84. [Crossref] [PubMed]
- Pandey P, Khan F, Seifeldin SA, et al. Targeting Wnt/β-Catenin Pathway by Flavonoids: Implication for Cancer Therapeutics. Nutrients 2023;15:2088. [Crossref] [PubMed]
- Taheriazam A, Bayanzadeh SD, Heydari Farahani M, et al. Non-coding RNA-based therapeutics in cancer therapy: An emphasis on Wnt/β-catenin control. Eur J Pharmacol 2023;951:175781. [Crossref] [PubMed]
- Nusse R, Clevers H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017;169:985-99. [Crossref] [PubMed]
- Jackstadt R, Hodder MC, Sansom OJ. WNT and β-Catenin in Cancer: Genes and Therapy. Annual Review of Cancer Biology 2020;4:177-96. [Crossref]
- Liu J, Xiao Q, Xiao J, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther 2022;7:3. [Crossref] [PubMed]
- Hu Z, Li L, Cheng P, et al. lncRNA MSC-AS1 activates Wnt/β-catenin signaling pathway to modulate cell proliferation and migration in kidney renal clear cell carcinoma via miR-3924/WNT5A. J Cell Biochem 2020;121:4085-93. [Crossref] [PubMed]
- Wang G, Zhang ZJ, Jian WG, et al. Novel long noncoding RNA OTUD6B-AS1 indicates poor prognosis and inhibits clear cell renal cell carcinoma proliferation via the Wnt/β-catenin signaling pathway. Mol Cancer 2019;18:15. [Crossref] [PubMed]
- Menon SS, Guruvayoorappan C, Sakthivel KM, et al. Ki-67 protein as a tumour proliferation marker. Clin Chim Acta 2019;491:39-45. [Crossref] [PubMed]
- González-Magaña A, Blanco FJ. Human PCNA Structure, Function and Interactions. Biomolecules 2020;10:570. [Crossref] [PubMed]
- Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 2019;20:69-84. [Crossref] [PubMed]
- Lachat C, Peixoto P, Hervouet E. Epithelial to Mesenchymal Transition History: From Embryonic Development to Cancers. Biomolecules 2021;11:782. [Crossref] [PubMed]
- Huang Y, Hong W, Wei X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J Hematol Oncol 2022;15:129. [Crossref] [PubMed]
- Li Q, Lai Q, He C, et al. RUNX1 promotes tumour metastasis by activating the Wnt/β-catenin signalling pathway and EMT in colorectal cancer. J Exp Clin Cancer Res 2019;38:334. [Crossref] [PubMed]
- Barth DA, Drula R, Ott L, et al. Circulating Non-coding RNAs in Renal Cell Carcinoma-Pathogenesis and Potential Implications as Clinical Biomarkers. Front Cell Dev Biol 2020;8:828. [Crossref] [PubMed]
- Ren S, Wang F, Shen J, et al. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 derived miniRNA as a novel plasma-based biomarker for diagnosing prostate cancer. Eur J Cancer 2013;49:2949-59. [Crossref] [PubMed]
- Wu Y, Wang YQ, Weng WW, et al. A serum-circulating long noncoding RNA signature can discriminate between patients with clear cell renal cell carcinoma and healthy controls. Oncogenesis 2016;5:e192. [Crossref] [PubMed]
- Wan B, Liu B, Huang Y, et al. Prognostic value of immune-related genes in clear cell renal cell carcinoma. Aging (Albany NY) 2019;11:11474-89. [Crossref] [PubMed]
- Yuan J, Yuan G. SEMA3G, downregulated by ncRNAs, correlates with favorable prognosis and tumor immune infiltration in kidney renal clear cell carcinoma. Aging (Albany NY) 2023;15:13944-60. [Crossref] [PubMed]
- Yao J, Chen Y, Wang Y, et al. Decreased expression of a novel lncRNA CADM1-AS1 is associated with poor prognosis in patients with clear cell renal cell carcinomas. Int J Clin Exp Pathol 2014;7:2758-67. [PubMed]
- Qian M, Zheng JL, Kang N, et al. Down-regulation of long noncoding RNA PGM5-AS1 correlates with tumor progression and predicts poor prognosis in clear cell renal cell carcinoma. Eur Rev Med Pharmacol Sci 2019;23:10685-90. [PubMed]
- Liu Y, Cheng G, Huang Z, et al. Long noncoding RNA SNHG12 promotes tumour progression and sunitinib resistance by upregulating CDCA3 in renal cell carcinoma. Cell Death Dis 2020;11:515. [Crossref] [PubMed]
- Li D, Li C, Chen Y, et al. LncRNA HOTAIR induces sunitinib resistance in renal cancer by acting as a competing endogenous RNA to regulate autophagy of renal cells. Cancer Cell Int 2020;20:338. [Crossref] [PubMed]
- Shen H, Luo G, Chen Q. Long noncoding RNAs as tumorigenic factors and therapeutic targets for renal cell carcinoma. Cancer Cell Int 2021;21:110. [Crossref] [PubMed]
- Polovic M, Dittmar S, Hennemeier I, et al. Identification of a novel lncRNA induced by the nephrotoxin ochratoxin A and expressed in human renal tumor tissue. Cell Mol Life Sci 2018;75:2241-56. [Crossref] [PubMed]


