Known for its extraordinariness, magnesium is the fourth most abundant mineral in the body. (1, 2) In the earth’s crust, magnesium is the eighth most abundant. It is the most abundant when water bodies are accounted for. In salt water, magnesium averages 1290 parts per million (ppm). (3)
Figure 1: Magnesium food sources. (4)
Magnesium (Mg)
-
- Lightest metal element (1.738 g/cm3) (20℃)
- Atomic number: 12
- Element category: Alkaline metal (3, 5)
Figure 2: Magnesium, an element of the periodic table, is displayed. (6)
History of Magnesium
Figure 3: Picture of magnesium. (3):brightness(10):contrast(5):no_upscale():format(webp)/CSIRO_ScienceImage_2893_Crystalised_magnesium-5c4dce4346e0fb0001a8e7ca.jpg)
In 1755, magnesium became known as one of the elements. The name came from Magnesia, the Greek word for a Thessaly district where magnesium was first found. (5) In 1618, farmer Henry Wicker tried giving water to his cows but the cows refused because the water tasted bitter. Henry noticed the water appeared to heal scratches and rashes. This took place in Epsom, England, and this then became widely used and was later recognized as magnesium sulfate, MgSO4, commonly known as Epsom salts. (7)
Figure 4: Epsom salts, magnesium sulfate, are used in baths. (8)

Magnesium Sources
- Nuts
- Cereals
- Seafood
- Meats (5)
Figure 5: Sources of magnesium. (2)
Magnesium production:
- Dolomite and magnesite ore
- Magnesium chloride containing salt brines (3)
Uses
-
- Antacids or cathartics
- They contain magnesium citrate, oxide, sulfate, hydroxide, and carbonate
- Antidote for poisoning
- Magnesium hydroxide (milk of magnesia)
- Inflammation relief
- Magnesium sulfate applied topically
- Seizure treatment for eclampsia in pregnancy and acute nephritis
- Administration of magnesium sulfate parenterally (5)
- Antacids or cathartics
Biotransformation
In microbial cells, magnesium is needed for cell metabolism as one of the macronutrients. Magnesium is present in optimal quantities in soils. Magnesium together with calcium, sulfur, potassium, and iron is crucial to cell synthesis. (9)
Toxicokinetics
- Oral magnesium
- Absorption occurs mostly in the small intestine
- Magnesium is also absorbed by the colon
- Absorption occurs mostly in the small intestine
- Competitive absorption
- Magnesium competes with calcium
- When there is excess calcium, magnesium absorption gets partially inhibited
- Magnesium competes with calcium
- Levels of magnesium in serum
- Constant
- Excretion
- Occurs via the digestive tract in bile and juices of pancreas and intestine
- 60% to 65% of magnesium in the body is in bone
- 27% of magnesium is in muscle
- 6% to 7% of magnesium is in other organs
- 1% of magnesium is in the fluid (extracellular fluid)
- Magnesium gets filtered by the glomeruli
- Approximately 95% gets reabsorbed–this contributes to the homeostasis maintenance (5)
Carcinogenicity
Magnesium supplementation is known to inhibit carcinogenesis, and magnesium deficiency contributes to increasing the incidence of cancer. When magnesium is administered parenterally with a carcinogen, local anticarcinogenic effects result (in comparison to zinc where zinc produces systemic effects). Magnesium supplementation reduces the binding of the carcinogen to cells and DNA, and such mechanisms may include metal-carcinogen interactions at target cells’ enzymatic and regulatory sites. Studies show magnesium to have stimulatory effects on the host immune system. (10)
Mechanism of Action
In the intracellular fluids, magnesium is the second most abundant, and it participates in many enzymatic activities, neurochemical transmission, and muscular excitability. As magnesium sulfate, it causes reductions in striated muscle contractions, and it reduces acetylcholine release at myoneural junction, causing blockage of peripheral neuromuscular transmission. Another action by magnesium is that it inhibits the influx of calcium through channels, resulting in magnesium’s relaxant action on vascular smooth muscle. (11)
Below is a brief video on the mechanism of action of magnesium as well as its uses in the clinical setting.
Video 1: Magnesium’s mechanism of action as well as its clinical uses. (18)
Target Organs
As magnesium oxide, the target organs are eyes and respiratory system. (12) As magnesium sulfate injection, the possible target organs are the gastrointestinal tract, nervous system, cardiovascular system, and heart. (13)
Signs and Symptoms of Toxicity
Industrial exposures
- Metal fume fever through inhalation of magnesium oxide that’s recently generated
- Similar to zinc oxide (5)
Nonoccupational exposures
- Persons with severe renal failure can suffer from magnesium toxicity through ingestion of antacids and other magnesium-containing drugs (5)
Signs and symptoms
- Nausea
- Vomiting
- Hypotension
- Electrocardiograph abnormalities
- Central nervous system effects
- Coma
- Systolic cardiac arrest (5)
Below talks about magnesium sulfate being used as a drug to help pregnant women, and it explains what to look for in possible magnesium sulfate toxicity.

Figure 6: Magnesium sulfate use in pregnancy and its toxicity. (19)
Genetic Susceptibility or Heritable Traits
Hypomagnesemia has been shown to be associated with several single nucleotide polymorphisms (SNPs) along with traits linked to serum magnesium levels which include:
- Kidney function
- Fasting glucose
- Bone mineral density (14)
There are common variants at six genomic regions, listed below, that are associated with serum magnesium levels at genome-wide significant levels.
- MUC1
- ATP2B1
- DCDC5
- TRPM6
- SHROOM3
- MDS1 (14)
Treatments
How to counteract magnesium toxicity
- Infusion with calcium (5)
Biomarkers
Biomarkers indicating magnesium status exist, and there is a total of 20 of them. The majority are for magnesium supplementation, and the rest are for magnesium depletion. Serum/plasma magnesium concentration, red blood cell (RBC) concentration, and urinary magnesium excretion are in response to alterations in diet. Those biomarkers appear to be useful in magnesium status of the general population. (15)
Essentiality and Deficiency
Magnesium is an essential metal in nutrition in which it participates in many crucial cellular reactions. (5)
- Enzyme cofactor (for many enzymes), meaning it is a “helper molecule” in biochemical reactions (5, 2)
- For the seven important enzymes in the glycolytic cycle, the divalent magnesium is required
- Enzymes containing magnesium
- Used in citric acid cycle
- Beta-oxidation of fatty acids (5)
Magnesium deficiency can result from the following:
- Malabsorption syndromes
- Renal dysfunction
- Endocrine disorders (5)
Magnesium deficiency can cause the following:
- Neuromuscular irritability
- Frank tetany
- Convulsions
- Inflammatory syndrome induction
- Be a risk factor for:
- Diabetes mellitus
- Hypertension
- Hyperlipidemia
- Ischemic heart disease (5)
To determine the presence of a magnesium deficiency, a blood test may be taken. (1) Magnesium levels may be measured in the serum where 0.79 millimoles per liter or lower is considered to be low in magnesium (and a high magnesium level measures at 0.90 millimoles per liter or above). (4)
Below are also associated with not enough magnesium in the body:
- Cramps
- Muscle pain
- Muscle spasms
- Brittle bone
- Fatigue
- Anxiety
- Migraines
- Mood swings
- Digestive or malabsorption issues (1)
The below image covers the symptoms of severe magnesium deficiency.
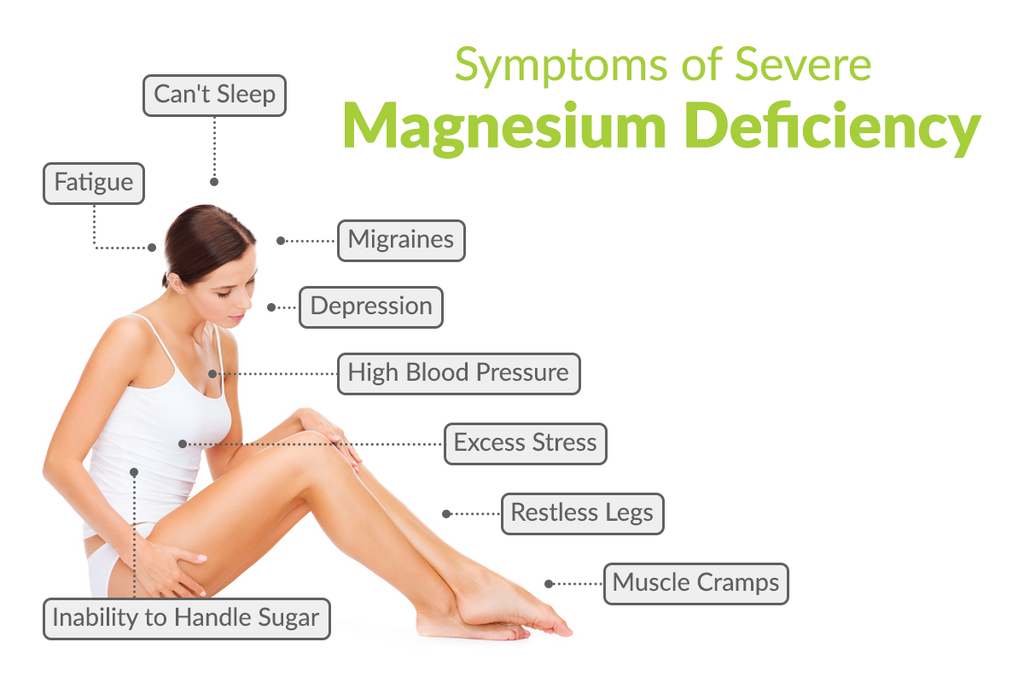
Figure 7: What severe magnesium deficiency looks like. (17)
To ameliorate, magnesium supplementation can derive benefits. It may be administered intravenously or orally. (5)
How prevalent is this deficiency though? Approximately 50-70% of American adults are deficient because they do not ingest enough of what’s recommended for their daily dose of magnesium. (2, 1)
Below is a table of what is the recommended daily amount of magnesium based on age, gender, pregnant, and lactating.
Table 1: Recommended daily amounts of magnesium. (16)
Thank you for reading about magnesium!
Figure 6: Magnesium dietary sources (1)
References:
- Signs Your Body Needs More Magnesium [Internet]. Pharmacy Solutions 223. 2018 [cited 2019May28]. Available from: https://rxcompound.com/10-signs-your-body-needs-more-magnesium/
- 10 Evidence-Based Health Benefits of Magnesium [Internet]. Healthline. Healthline Media; [cited 2019May28]. Available from: https://www.healthline.com/nutrition/10-proven-magnesium-benefits#section1
- Bell T. Everything You Want to Know About Magnesium [Internet]. The Balance. The Balance; 2019 [cited 2019Jun9]. Available from: https://www.thebalance.com/metal-profile-magnesium-2340142
- Sandoiu A. Too much or too little magnesium can raise dementia risk [Internet]. Medical News Today. MediLexicon International; 2017 [cited 2019May28]. Available from: https://www.medicalnewstoday.com/articles/319481.php
- Tokar EJ, Boyd WA, Freedman JH, Waalkes MP. Toxic Effects of Metals. In: Klaassen CD. eds. Casarett and Doull’s Toxicology: The Basic Science of Poisons, Eighth Edition New York, NY: McGraw-Hill; 2013. http://accesspharmacy.mhmedical.com.proxy.lib.ohio-state.edu/content.aspx?bookid=958§ionid=53483748. Accessed May 25, 2019.
- Treefrog Inc. Magnesium: A History [Internet]. Mvua Magnesium. 2018 [cited 2019Jun9]. Available from: https://www.mvuahealth.ca/blog-magnesium-a-history
- Administrator. History [Internet]. Magnesium… Morgo Magnesium Limited; [cited 2019Jun9]. Available from: https://www.magnesiumsquare.com/index.php?option=com_content&view=article&id=13&Itemid=31
- Romero VR, Christ-follower VRRA. How To Take A Detox Bath | Vanessa Rae Romero [Internet]. Healthy Living How To. 2019 [cited 2019Jun9]. Available from: https://healthylivinghowto.com/detoxification-part-i-healing-waters/
- Session II-1: Biotransformation/Biodegradation overview. [cited 2019Jun9]. Available from: https://www.cs.montana.edu/webworks/projects/oldjunk/Bioremediation/Bioremediation_save/Sessions/SessionII-1.htm
- Kasprzak KS, Waalkes MP. The role of calcium, magnesium, and zinc in carcinogenesis [Internet]. Advances in experimental medicine and biology. U.S. National Library of Medicine; 1986 [cited 2019Jun9]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/3591535
- Magnesium sulfate [Internet]. DrugBank. [cited 2019Jun9]. Available from: https://www.drugbank.ca/drugs/DB00653
- CDC – NIOSH Pocket Guide to Chemical Hazards – Magnesium oxide fume [Internet]. Centers for Disease Control and Prevention. Centers for Disease Control and Prevention; [cited 2019Jun9]. Available from: https://www.cdc.gov/niosh/npg/npgd0374.html
- Hospira. Material Safety Data Sheet | Product Name: Magnesium Sulfate Injection, USP. Old Saybrook: savelives.com; 2005. Available from: https://www.savelives.com/media/pdf/Magnesium.pdf
- Meyer TE, Germaine C. Verwoert S-JH, Glazer NL, Smith AV, Rooij FJAvan, Ehret GB, et al. Genome-Wide Association Studies of Serum Magnesium, Potassium, and Sodium Concentrations Identify Six Loci Influencing Serum Magnesium Levels [Internet]. PLOS Genetics. Public Library of Science; 2010 [cited 2019Jun9]. Available from: https://journals.plos.org/plosgenetics/article?id=10.1371/journal.pgen.1001045
- Witkowski M, Hubert J, Mazur A. Methods of assessment of magnesium status in humans: a systematic review [Internet]. Magnesium research. U.S. National Library of Medicine; 2011 [cited 2019Jun9]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/22064327
- Office of Dietary Supplements – Magnesium [Internet]. NIH Office of Dietary Supplements. U.S. Department of Health and Human Services; [cited 2019May28]. Available from: https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/
- Magnesium – The Miracle Mineral [Internet]. Nu U Nutrition. [cited 2019Jul29]. Available from: https://nuunutrition.com/blogs/news/magnesium-the-miracle-mineral
- Anaesthesiologist T. [Internet]. YouTube. YouTube; 2018 [cited 2019Jul29]. Available from: https://www.youtube.com/watch?v=HYLWBKki9gA
- Tr-I-Life. [Internet]. Tr. 2012 [cited 2019Jul29]. Available from: https://tr-i-life.tumblr.com/post/31788794806/magnesium-sulfate-toxicitypih

Very informative. Enjoyed reading this.
Mighty Magnesium
Through the years, you probably collected a few different supplements and vitamins but have forgotten about them for one reason or another. There’s this one, in particular, you should give second thoughts, especially if you’ve been feeling sluggish lately: magnesium.
MAGNESIUM IS IMPORTANT FOR HEALTH
Magnesium is present in more foods and supplements than you know. It’s a mineral found in some medicines like Milk of Magnesia, fruits, and dietary supplements. You could consume breakfast cereals, oatmeal, and magnesium rich foods containing fiber. But what does magnesium do for the body?
It supports the muscles, blood pressure and energy levels, besides nerve function. Magnesium promotes potassium and calcium in the bloodstream. The mineral is necessary to have a healthy heart muscle, bone formation, and to stabilize blood sugar levels and moods. If you have migraine headaches, diabetes or other illnesses, you may have suffered from a magnesium-deficiency.
Here is a great article I read about magnesium- https://www.thegoodmoodco.com/journal/might-magnesium
Magnesium (Mg) is the lightest structural metal and one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. Magnesium is one of the elements required for all cellular life, and its derivatives are widely employed in construction and medicine. Do you know magnesium ribbon should be cleaned before burning in air. But Why? Let us know