What is Clostridium difficile (C. difficile) Infection and How to Use the Clostridium difficile Rapid Test Kit?

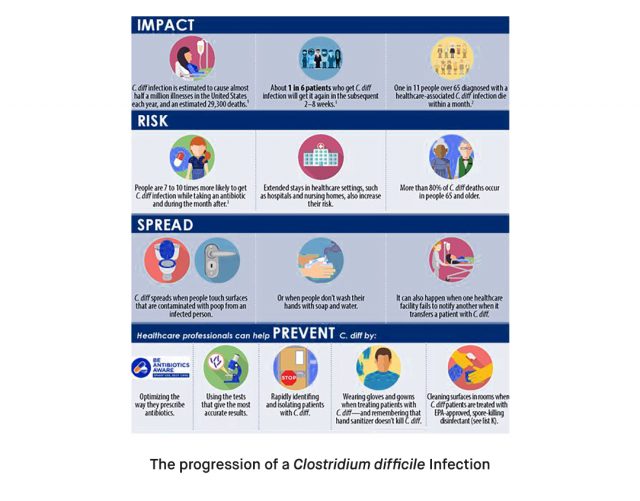
Clostridium difficile (syn. Clostridioides difficile) is a life-threatening gram-positive bacterium that causes diarrheal infections, leading to serious colon infections and even colon cancer, especially in older people [1]. Clostridium difficile is an important emerging human pathogen; according to the CDC, in 2017, there were 223,900 cases in hospitalized patients and 12,800 deaths in the United States [2]. Continue reading to learn more about Clostridium difficile, significant symptoms requiring attention, various testing procedures, and how to treat Clostridium difficile infection.
What is Clostridium difficile (C. difficile)?

Anaerobic, gram-positive, spore-forming, toxin-producing Clostridium difficile spreads among people via the fecal-oral pathway. It was long believed that the bacillus and humans shared a commensal relationship, but C. difficile has now emerged as a significant gastrointestinal pathogen with a global distribution. The most often reported nosocomial pathogen in the US is C. difficile. In 2011, a surveillance study found 453,000 instances of C. difficile infection and 29,000 fatalities attributed to the illness; around a quarter of those infections were acquired in the community. Hospitalization costs resulting from nosocomial C. difficile infection more than treble, adding $1.5 billion to annual spending in the US [3]. The prevalence and severity of C. difficile infections have risen globally over the past ten years, making them one of the most prevalent diseases acquired in hospitals [4].
What are the symptoms of a Clostridium difficile (C. difficile) Infection?

Severe C. difficile infections can lead to dehydration, which necessitates hospitalization. In addition, due to C. difficile, the colon may become inflamed and may develop raw tissue patches that may bleed or generate pus. Typically, signs and symptoms appear 5 to 10 days after beginning an antibiotic treatment. Signs and symptoms may include watery diarrhea as often as 10 to 15 times a day, abdominal cramping and pain, which may be severe, rapid heart rate, dehydration, fever, nausea, increased white blood cell count, kidney failure, loss of appetite, swollen abdomen, weight loss and blood or pus in the stool.
The clinical picture and symptoms are very diverse, and these varying degrees of symptoms require urgent medical attention. Otherwise, these symptoms can progress to the most severe form of colitis, which can be life-threatening and fatal [4].
What is the Clostridium difficile Rapid Test Kit?

Severe C. difficile infections can lead to dehydration, which necessitates hospitalization. In addition, due to C. difficile, the colon may become inflamed and may develop raw tissue patches that may bleed or generate pus. Typically, signs and symptoms appear 5 to 10 days after beginning an antibiotic treatment. Signs and symptoms may include watery diarrhea as often as 10 to 15 times a day, abdominal cramping and pain, which may be severe, rapid heart rate, dehydration, fever, nausea, increased white blood cell count, kidney failure, loss of appetite, swollen abdomen, weight loss and blood or pus in the stool.
The clinical picture and symptoms are very diverse, and these varying degrees of symptoms require urgent medical attention. Otherwise, these symptoms can progress to the most severe form of colitis, which can be life-threatening and fatal [4].
How to Use the Clostridium difficile Rapid Test Kit?
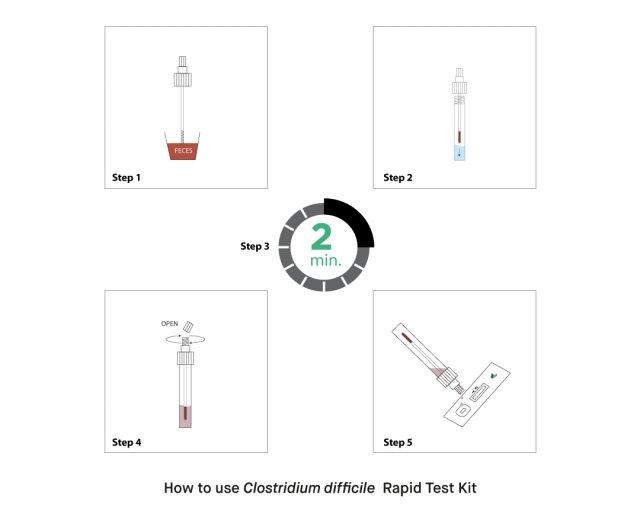
In the use of the Clostridium difficile rapid test kit, fecal samples are usually used as specimens. A small amount of fecal samples is first collected with the apparatus used in the kit. The sample is then mixed with the extraction buffer for 2 minutes. The test cassette is then removed from the package and placed on a flat surface. Finally, 3-4 drops of the sample are dropped into the well on the cassette. After 15 minutes, if both the C and T lines on the cassette are visible, the test is positive. If the test line is not visible, the test is negative. However, if the C line is not visible, the test is invalid and needs to be repeated.
How to Treat Clostridium difficile Infection?
If feasible, patients who are receiving antibiotics when symptoms start should discontinue. This interruption in antibiotic treatment may occasionally cause the symptoms to go away independently. Antibiotics that can destroy C. difficile spores will need to be used to treat patients who do not improve after stopping broad-spectrum antibiotics. Vancomycin is primarily used to treat primary infections, with a typical dose of 125 mg every 6 hours [5].
A fecal microbiota transplant (FMT) may be an option for patients who do not respond to conventional antibiotic treatment. With recurrent CDI (Clostridium difficile Infection), healthcare professionals can transfer feces from a healthy individual to a patient’s colon. With a 93% cure rate, this procedure is the most effective therapy for severe CDI. Recurrence rates of CDI in patients receiving an FMT are typically low, hovering around 19%, making it an excellent option for treating instances of persistent CDI. In certain instances, the medication might cause flare-ups of inflammatory bowel disease [6]. As FMT has only been FDA-approved since 2011 and only a small number of operations have been carried out, its long-term implications remain unknown. The contaminated portion of the colon can be removed to treat CDI if transplantation is not a possibility [7][8].
REFERENCES
[1] Smits, W. K., Lyras, D., Lacy, D. B., Wilcox, M. H., & Kuijper, E. J. (2016). Clostridium difficile infection. Nature reviews Disease primers, 2(1), 1-20.
[2] “Clostridioides difficile Infection | HAI | CDC”. www.cdc.gov. 2020-01-02.
[3] Leffler, D. A., & Lamont, J. T. (2015). Clostridium difficile infection. New England Journal of Medicine, 372(16), 1539-1548.
[4] Czepiel, J., Dróżdż, M., Pituch, H., Kuijper, E. J., Perucki, W., Mielimonka, A., … & Biesiada, G. (2019). Clostridium difficile infection. European Journal of Clinical Microbiology & Infectious Diseases, 38, 1211-1221.
[5] “Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA)”. Clinical Infectious Diseases. 66 (7): e1–e48. 2018-03-19.
[6] Chen, Yu-Gen; Zheng, Xiao; Zhou, Jin-Yong; Wu, Jing; Jiang, Feng; Zhang, Dan; Zhou, Qun; Chen, Tuo (2018-05-25). “Effect of Faecal Microbiota Transplantation for Treatment of Clostridium difficile Infection in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Cohort Studies”. Journal of Crohn’s and Colitis. 12 (6): 710–717.
[7] Surawicz, Christina M; Brandt, Lawrence J; Binion, David G; Ananthakrishnan, Ashwin N; Curry, Scott R; Gilligan, Peter H; McFarland, Lynne V; Mellow, Mark; Zuckerbraun, Brian S (2013-02-26). “Guidelines for Diagnosis, Treatment and Prevention of Clostridium difficile Infections”. The American Journal of Gastroenterology. 108 (4): 478–498.