Study of the Relationship between Sigma Receptor Expression Levels and Some Common Sigma Ligand Activity in Cancer Using Human Cancer Cell Lines of the NCI-60 Cell Line Panel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Western Blot Analysis
2.3. Cell Viability Assay
2.4. Statistical Analysis
3. Results
3.1. σ1 Receptor, PGRMC1, and σ2/TMEM97 Were Heterogeneously Expressed in 23 Human Cancer Cell Lines
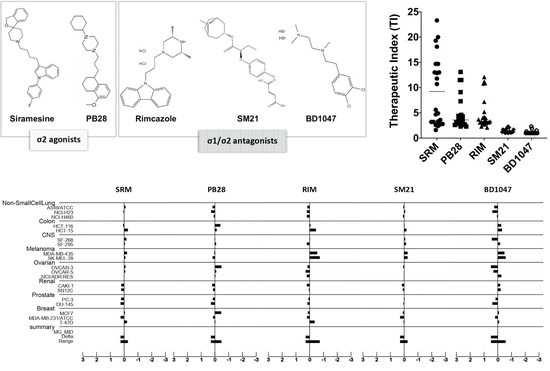
3.2. In Vitro Antiproliferative Efficacy of Sigma Ligands
3.3. Sigma Ligands’ Sensitivity Was Not Correlated to Their Activity as Either Agonists or Antagonists
3.4. Sigma Receptors Expression Levels Did Not Appear to Correlate with the Sigma Ligands’ Antiproliferative Effect
3.5. Putative Sigma Ligands’ Mechanism of Action: Prediction Using the COMPARE Algorithm
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, W.R.; Eades, C.G.; Thompson, J.A.; Huppler, R.E.; Gilbert, P.E. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976, 197, 517–532. [Google Scholar] [PubMed]
- Hanner, M.; Moebius, F.F.; Flandorfer, A.; Knaus, H.G.; Striessnig, J.; Kempner, E.; Glossmann, H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc. Natl. Acad. Sci. USA 1996, 93, 8072–8077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kekuda, R.; Prasad, P.D.; Fei, Y.-J.; Leibach, F.H.; Ganapathy, V. Cloning and Functional Expression of the Human Type 1 Sigma Receptor (hSigmaR1). Biochem. Biophys. Res. Commun. 1996, 229, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Su, T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Zeng, C.; Chu, W.; Pan, F.; Rothfuss, J.M.; Zhang, F.; Tu, Z.; Zhou, D.; Zeng, D.; Vangveravong, S.; et al. Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nat. Commun. 2011, 2, 380. [Google Scholar] [CrossRef]
- Alon, A.; Schmidt, H.R.; Wood, M.D.; Sahn, J.J.; Martin, S.F.; Kruse, A.C. Identification of the gene that codes for the sigma2 receptor. Proc. Natl. Acad. Sci. USA 2017, 114, 7160–7165. [Google Scholar] [CrossRef] [Green Version]
- Schmit, K.; Michiels, C. TMEM Proteins in Cancer: A Review. Front. Pharm. 2018, 9, 1345. [Google Scholar] [CrossRef] [Green Version]
- Riad, A.; Zeng, C.; Weng, C.-C.; Winters, H.; Xu, K.; Makvandi, M.; Metz, T.; Carlin, S.; Mach, R.H. Sigma-2 Receptor/TMEM97 and PGRMC-1 Increase the Rate of Internalization of LDL by LDL Receptor through the Formation of a Ternary Complex. Sci. Rep. 2018, 8, 16845. [Google Scholar] [CrossRef]
- Schmidt, H.R.; Kruse, A.C. The Molecular Function of sigma Receptors: Past, Present, and Future. Trends Pharm. Sci. 2019, 40, 636–654. [Google Scholar] [CrossRef]
- Georgiadis, M.O.; Karoutzou, O.; Foscolos, A.S.; Papanastasiou, I. Sigma Receptor (sigmaR) Ligands with Antiproliferative and Anticancer Activity. Molecules 2017, 22, 1408. [Google Scholar] [CrossRef] [Green Version]
- Covell, D.G.; Huang, R.; Wallqvist, A. Anticancer medicines in development: Assessment of bioactivity profiles within the National Cancer Institute anticancer screening data. Mol. Cancer Ther. 2007, 6, 2261–2270. [Google Scholar] [CrossRef] [Green Version]
- Søby, K.K.; Mikkelsen, J.D.; Meier, E.; Thomsen, C. Lu 28-179 labels a sigma(2)-site in rat and human brain. Neuropharmacology 2002, 43, 95–100. [Google Scholar] [CrossRef]
- Berardi, F.; Colabufo, N.A.; Giudice, G.; Perrone, R.; Tortorella, V.; Govoni, S.; Lucchi, L. New sigma and 5-HT1A receptor ligands: Omega-(tetralin-1-yl)-n-alkylamine derivatives. J. Med. Chem. 1996, 39, 176–182. [Google Scholar] [CrossRef]
- Ferris, R.M.; Tang, F.L.; Chang, K.J.; Russell, A. Evidence that the potential antipsychotic agent rimcazole (BW 234U) is a specific, competitive antagonist of sigma sites in brain. Life Sci. 1986, 38, 2329–2337. [Google Scholar] [CrossRef]
- Ghelardini, C.; Galeotti, N.; Gualtieri, F.; Bellucci, C.; Manetti, D.; Giotti, A.; Malmberg-Aiello, P.; Galli, A.; Bartolini, A. Antinociceptive profile of 3-alpha-tropanyl 2-(4-Cl-phenoxy)butyrate (SM-21) [corrected]: A novel analgesic with a presynaptic cholinergic mechanism of action. J. Pharm. Exp. 1997, 282, 430–439. [Google Scholar]
- Matsumoto, R.R.; Bowen, W.D.; Tom, M.A.; Vo, V.N.; Truong, D.D.; De Costa, B.R. Characterization of two novel sigma receptor ligands: Antidystonic effects in rats suggest sigma receptor antagonism. Eur. J. Pharm. 1995, 280, 301–310. [Google Scholar] [CrossRef]
- Developmental Therapeutics Program. Available online: https://dtp.cancer.gov/databases_tools/compare.htm (accessed on 24 March 2020).
- Mahaira, L.G.; Tsimplouli, C.; Sakellaridis, N.; Alevizopoulos, K.; Demetzos, C.; Han, Z.; Pantazis, P.; Dimas, K. The labdane diterpene sclareol (labd-14-ene-8, 13-diol) induces apoptosis in human tumor cell lines and suppression of tumor growth in vivo via a p53-independent mechanism of action. Eur. J. Pharmacol. 2011, 666, 173–182. [Google Scholar] [CrossRef]
- Holbeck, S.L.; Collins, J.M.; Doroshow, J.H. Analysis of Food and Drug Administration-approved anticancer agents in the NCI60 panel of human tumor cell lines. Mol. Cancer 2010, 9, 1451–1460. [Google Scholar] [CrossRef] [Green Version]
- Allen, L.M.; Creaven, P.J. Inhibition of Macromolecular Biosynthesis in Cultured L1210 Mouse Leukemia Cells by Thalicarpine (NSC 68075). Cancer Res. 1973, 33, 3112–3116. [Google Scholar]
- NCI Drug Dictionary. Available online: https://www.cancer.gov/publications/dictionaries/cancer-drug (accessed on 26 December 2020).
- Wilson, J.J.; Lippard, S.J. In vitro anticancer activity of cis-diammineplatinum(II) complexes with β-diketonate leaving group ligands. J. Med. Chem. 2012, 55, 5326–5336. [Google Scholar] [CrossRef] [Green Version]
- Jackson, R.C.; Taylor, G.A.; Harrap, K.R. Aspects of the biochemical pharmacology of cytembena. Neoplasma 1975, 22, 259–268. [Google Scholar]
- Isah, T. Anticancer Alkaloids from Trees: Development into Drugs. Pharm. Rev. 2016, 10, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Slavik, M.; Blanc, O.; Davis, J. Spirogermanium: A new investigational drug of novel structure and lack of bone marrow toxicity. Investig. New Drugs 1983, 1, 225–234. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, Y.; Huang, L.; Jia, X.; Zhang, Q.; Zhang, X.; Tang, G.; Liu, W. Cloning and Characterization of the Tetrocarcin A Gene Cluster from Micromonospora chalcea NRRL 11289 Reveals a Highly Conserved Strategy for Tetronate Biosynthesis in Spirotetronate Antibiotics. J. Bacteriol. 2008, 190, 6014–6025. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Hayashi, T.; Hayashi, E.; Su, T.P. Sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS ONE 2013, 8, e76941. [Google Scholar] [CrossRef] [Green Version]
- Leithner, K.; Wohlkoenig, C.; Stacher, E.; Lindenmann, J.; Hofmann, N.A.; Gallé, B.; Guelly, C.; Quehenberger, F.; Stiegler, P.; Smolle-Jüttner, F.M.; et al. Hypoxia increases membrane metallo-endopeptidase expression in a novel lung cancer ex vivo model—role of tumor stroma cells. BMC Cancer 2014, 14, 40. [Google Scholar] [CrossRef] [Green Version]
- Spruce, B.A.; Campbell, L.A.; McTavish, N.; Cooper, M.A.; Appleyard, M.V.; O’Neill, M.; Howie, J.; Samson, J.; Watt, S.; Murray, K.; et al. Small molecule antagonists of the sigma-1 receptor cause selective release of the death program in tumor and self-reliant cells and inhibit tumor growth in vitro and in vivo. Cancer Res. 2004, 64, 4875–4886. [Google Scholar] [CrossRef] [Green Version]
- Schrock, J.M.; Spino, C.M.; Longen, C.G.; Stabler, S.M.; Marino, J.C.; Pasternak, G.W.; Kim, F.J. Sequential cytoprotective responses to Sigma1 ligand-induced endoplasmic reticulum stress. Mol. Pharm. 2013, 84, 751–762. [Google Scholar] [CrossRef] [Green Version]
- Brune, S.; Schepmann, D.; Lehmkuhl, K.; Frehland, B.; Wünsch, B. Characterization of Ligand Binding to the σ1 Receptor in a Human Tumor Cell Line (RPMI 8226) and Establishment of a Competitive Receptor Binding Assay. Assay Drug Dev. Technol. 2011, 10, 365–374. [Google Scholar] [CrossRef]
- Han, K.Y.; Gu, X.; Wang, H.R.; Liu, D.; Lv, F.Z.; Li, J.N. Overexpression of MAC30 is associated with poor clinical outcome in human non-small-cell lung cancer. Tumour Biol. 2013, 34, 821–825. [Google Scholar] [CrossRef]
- Moparthi, S.B.; Arbman, G.; Wallin, A.; Kayed, H.; Kleeff, J.; Zentgraf, H.; Sun, X.F. Expression of MAC30 protein is related to survival and biological variables in primary and metastatic colorectal cancers. Int. J. Oncol. 2007, 30, 91–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, H.; Gui, X.H.; Lin, X.B.; Chen, R.H.; Cai, H.R.; Fen, Y.; Sheng, Y.L. Prognostic Value of MAC30 Expression in Human Pure Squamous Cell Carcinomas of the Lung. Asian Pac. J. Cancer Prev. 2016, 17, 2705–2710. [Google Scholar] [PubMed]
- Aydar, E.; Onganer, P.; Perrett, R.; Djamgoz, M.B.; Palmer, C.P. The expression and functional characterization of sigma (sigma) 1 receptors in breast cancer cell lines. Cancer Lett. 2006, 242, 245–257. [Google Scholar] [CrossRef]
- Vilner, B.J.; John, C.S.; Bowen, W.D. Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer Res. 1995, 55, 408–413. [Google Scholar] [PubMed]
- Neubauer, H.; Adam, G.; Seeger, H.; Mueck, A.O.; Solomayer, E.; Wallwiener, D.; Cahill, M.A.; Fehm, T. Membrane-initiated effects of progesterone on proliferation and activation of VEGF in breast cancer cells. Climacteric 2009, 12, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Lucke-Wold, B.P.; Mookerjee, S.A.; Cavendish, J.Z.; Robson, M.J.; Scandinaro, A.L.; Matsumoto, R.R. Role of sigma-1 receptors in neurodegenerative diseases. J. Pharm. Sci. 2015, 127, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Weng, C.-C.; Schneider, M.E.; Puentes, L.; Riad, A.; Xu, K.; Makvandi, M.; Jin, L.; Hawkins, W.G.; Mach, R.H. TMEM97 and PGRMC1 do not mediate sigma-2 ligand-induced cell death. Cell Death Discov. 2019, 5, 58. [Google Scholar] [CrossRef] [Green Version]
- Crawford, K.W.; Bowen, W.D. Sigma-2 receptor agonists activate a novel apoptotic pathway and potentiate antineoplastic drugs in breast tumor cell lines. Cancer Res. 2002, 62, 313–322. [Google Scholar]
- Kashiwagi, H.; McDunn, J.E.; Simon, P.O.; Goedegebuure, P.S.; Xu, J.; Jones, L.; Chang, K.; Johnston, F.; Trinkaus, K.; Hotchkiss, R.S.; et al. Selective sigma-2 ligands preferentially bind to pancreatic adenocarcinomas: Applications in diagnostic imaging and therapy. Mol. Cancer 2007, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Rothfuss, J.; Zhang, J.; Chu, W.; Vangveravong, S.; Tu, Z.; Pan, F.; Chang, K.C.; Hotchkiss, R.; Mach, R.H. Sigma-2 ligands induce tumour cell death by multiple signalling pathways. Br. J. Cancer 2012, 106, 693–701. [Google Scholar] [CrossRef]
- Ostenfeld, M.S.; Fehrenbacher, N.; Høyer-Hansen, M.; Thomsen, C.; Farkas, T.; Jäättelä, M. Effective tumor cell death by sigma-2 receptor ligand siramesine involves lysosomal leakage and oxidative stress. Cancer Res. 2005, 65, 8975–8983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafner Česen, M.; Repnik, U.; Turk, V.; Turk, B. Siramesine triggers cell death through destabilisation of mitochondria, but not lysosomes. Cell Death Dis. 2013, 4, e818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Henson, E.S.; Chen, Y.; Gibson, S.B. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016, 7, e2307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, T.; Wang, S.; Zhao, Y.; Zhu, R.; Wang, W.; Sun, Y. Haloperidol, a sigma receptor 1 antagonist, promotes ferroptosis in hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2017, 491, 919–925. [Google Scholar] [CrossRef]











| CANCER TYPE | CELL LINES | SIRAMESINE | PB28 | RIMCAZOLE | SM21 | BD1047 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GI50 | TGI | LC50 | TI | GI50 | TGI | LC50 | TI | GI50 | TGI | LC50 | TI | GI50 | TGI | LC50 | TI | GI50 | TGI | LC50 | TI | ||
| NSCL | A549/ATCC | 3.6 | 7.7 | 11.7 | 3.3 | 34.2 | 66.8 | 99.3 | 2.9 | 29.2 | 61.0 | 92.7 | 3.2 | 84.2 | 100.0 | 100.0 | 1.2 | >100 | >100 | >100 | 1.0 |
| NCI-H23 | 4.7 | 7.9 | 11.2 | 2.4 | 42.9 | 87.0 | >100 | 2.3 | 31.9 | 62.8 | 93.7 | 2.9 | 65.2 | 100.0 | 100.0 | 1.5 | >100 | >100 | >100 | 1.0 | |
| NCI-H460 | 4.3 | 7.7 | 83.1 | 19.3 | 27.5 | 62.6 | 97.7 | 3.6 | 31.6 | 63.6 | 95.6 | 3.0 | 88.9 | 100.0 | 100.0 | 1.1 | >100 | >100 | >100 | 1.0 | |
| Colon | HCT-116 | 5.0 | 15.9 | 90.5 | 18.1 | 9.6 | 48.0 | 87.4 | 9.1 | 18.5 | 57.5 | 96.5 | 5.2 | 74.5 | 100.0 | 100.0 | 1.3 | 89.9 | >100 | >100 | 1.1 |
| HCT-15 | 2.4 | 7.9 | 13.5 | 5.6 | 17.9 | 48.6 | 79.4 | 4.4 | 7.5 | 38.5 | 82.1 | 10.9 | 53.7 | 100.0 | 100.0 | 1.9 | 77.8 | >100 | >100 | 1.3 | |
| CNS | SF-268 | 3.1 | 6.4 | 9.8 | 3.2 | 24.0 | 55.5 | 86.9 | 3.6 | 20.1 | 51.9 | 83.7 | 4.2 | 65.5 | 100.0 | 100.0 | 1.5 | >100 | >100 | >100 | 1.0 |
| SF-295 | 4.0 | 8.2 | 58.6 | 14.7 | 19.9 | 52.5 | 85.0 | 4.3 | 31.9 | 59.4 | 86.9 | 2.7 | 57.7 | 100.0 | 100.0 | 1.7 | >100 | >100 | >100 | 1.0 | |
| Melanoma | MDA-MB-435 | 2.8 | 5.9 | 9.0 | 3.2 | 25.6 | 56.6 | 87.7 | 3.4 | 6.0 | 10.7 | 72.5 | 12.1 | 44.0 | 94.2 | 100.0 | 2.3 | 51.7 | 10.1 | >100 | 1.9 |
| SK-MEL-28 | 3.6 | 6.7 | 0.8 | 2.7 | 33.4 | 59.9 | 86.3 | 2.6 | 3.9 | 7.2 | 28.4 | 7.2 | 44.6 | 80.1 | 100.0 | 2.2 | 42.6 | 86.8 | >100 | 2.3 | |
| Ovarian | OVCAR-3 | 3.6 | 7.6 | 71.9 | 20.0 | 8.2 | 47.5 | 94.0 | 11.5 | 28.8 | 61.3 | 93.8 | 3.3 | • | • | • | • | >100 | >100 | >100 | 1.0 |
| OVCAR-5 | 3.8 | 6.7 | 9.7 | 2.6 | 19.5 | 54.4 | 89.3 | 4.6 | 38.7 | 64.0 | 89.2 | 2.3 | • | • | • | • | >100 | >100 | >100 | 1.0 | |
| NCI/ADR-RES | 4.3 | 7.6 | >100 | 23.3 | 22.3 | 56.3 | 90.2 | 4.0 | 32.4 | 65.9 | 99.3 | 3.1 | 86.3 | 100.0 | 100.0 | 1.2 | >100 | >100 | >100 | 1.0 | |
| Kidney | CAKI-1 | 6.8 | 50.7 | >100 | 14.7 | 36.4 | 66.5 | 96.5 | 2.7 | 31.8 | 64.8 | 97.8 | 3.1 | 100.0 | 100.0 | 100.0 | 1.0 | >100 | >100 | >100 | 1.0 |
| SN12C | 6.9 | 36.2 | 89.3 | 13.0 | 31.5 | 60.2 | 89.0 | 2.8 | 22.6 | 56.3 | 90.0 | 4.0 | 91.9 | 100.0 | 100.0 | 1.1 | 98.5 | >100 | >100 | 1.0 | |
| Prostate | PC-3 | 7.0 | 35.9 | 90.5 | 12.9 | 32.5 | 59.3 | 86.0 | 2.6 | 24.6 | 31.2 | 84.3 | 3.4 | 73.9 | 100.0 | 100.0 | 1.4 | >100 | >100 | >100 | 1.0 |
| DU-145 | 7.7 | 40.6 | 90.8 | 11.8 | 44.3 | 77.9 | >100 | 2.3 | 28.8 | 58.7 | 88.5 | 3.1 | 82.3 | 100.0 | 100.0 | 1.2 | >100 | >100 | >100 | 1.0 | |
| Breast | MCF7 | 4.0 | 7.5 | 42.3 | 10.7 | 8.6 | 49.8 | 98.8 | 11.5 | 27.1 | 58.8 | 90.5 | 3.3 | 91.8 | 100.0 | 100.0 | 1.1 | >100 | >100 | >100 | 1.0 |
| MDA-MB-231 | 7.7 | 55.9 | >100 | 13.0 | 31.3 | 62.3 | 93.4 | 3.0 | 31.2 | 60.8 | 90.4 | 2.9 | 100.0 | 100.0 | 100.0 | 1.0 | >100 | >100 | >100 | 1.0 | |
| T-47D | 2.8 | 6.5 | 10.2 | 3.6 | 22.8 | 61.8 | >100 | 4.4 | 9.2 | 51.9 | 99.1 | 10.8 | 81.8 | 100.0 | 100.0 | 1.2 | >100 | >100 | >100 | 1.0 | |
| Pancreas | AsPC-1 | 1.7 | 5.0 | 8.3 | 4.9 | 7.8 | 47.6 | >100 | 13.1 | 7.2 | 39.2 | 80.4 | 11.2 | 58.6 | 100.0 | 100.0 | 1.7 | 65.7 | >100 | >100 | 1.5 |
| BxPC-3 | 2.0 | 5.6 | 9.3 | 4.7 | 12.8 | 53.1 | 93.4 | 7.3 | 22.1 | 55.2 | 88.3 | 4.0 | 81.5 | 100.0 | 100.0 | 1.2 | >100 | >100 | >100 | 1.0 | |
| MIAPaCa-2 | 3.1 | 5.9 | 8.7 | 2.8 | 28.8 | 58.5 | 88.1 | 3.1 | 4.5 | 6.9 | 9.3 | 2.1 | 45.8 | 71.1 | 96.4 | 2.1 | >100 | >100 | >100 | 1.0 | |
| PANC-1 | 4.0 | 7.0 | 6.3 | 1.6 | • | • | • | • | 22.6 | 52.9 | 83.3 | 3.7 | • | • | • | • | • | • | • | • | |
| MEAN | 4.3 | 15.3 | 45.0 | 9.2 | 24.6 | 58.8 | 92.4 | 5.0 | 22.3 | 49.6 | 83.3 | 4.9 | 73.6 | 97.3 | 99.8 | 1.4 | 92.1 | 361.1 | 100.0 | 1.1 | |
| Ligand | PCC | Target Vector | Mechanism of Action (MoA) |
|---|---|---|---|
| Siramesine | 0.601 | dihydro-5-azacytidine | DNA damage [21] |
| 0.519 | cyanomorpholino-ADR | Alkylating agent [22] | |
| 0.507 | cytembena | Inhibition of DNA and protein synthesis [23] | |
| 0.487 | anguidine | Inhibition of protein synthesis [21] | |
| 0.462 | caracemide | Inhibition of DNA synthesis [23] | |
| PB28 dihydrochloride | 0.724 | pibenzimol hydrochloride | Inhibition of DNA replication [21] |
| 0.561 | cytembena | Inhibition of DNA and protein synthesis [23] | |
| 0.514 | chloroquinoxaline sulfonamide | DNA damage [21] | |
| 0.501 | tamoxifen | Inhibition of DNA synthesis [23] | |
| 0.46 | 6-mercaptopurine | Inhibition of DNA synthesis [23] | |
| Rimcazole dihychloride | 0.884 | thalicarpine | Inhibition of DNA, RNA, and protein synthesis [24] |
| 0.687 | spirogermanium | Inhibition of protein synthesis [25] | |
| 0.541 | caracemide | Inhibition of DNA synthesis [21] | |
| 0.516 | tetrocarcin A sodium salt | Inhibition of mitochondrial function [26] | |
| 0.513 | semustine (methyl-CCNU) | Alkylating agent [21] | |
| SM21 maleate | 0.585 | anguidine | Inhibition of protein synthesis [21] |
| 0.523 | caracemide | Inhibition of DNA synthesis [21] | |
| 0.490 | rhizoxin | Antimitotic agent [21] | |
| 0.485 | tetrocarcin A sodium salt | Inhibit mitochondrial function [26] | |
| 0.466 | didemnin B | Inhibition of protein synthesis [21] | |
| BD1047 dihydrobromide | 0.618 | spirogermanium | Inhibition of DNA, RNA, and protein synthesis [25] |
| 0.618 | tamoxifen | Inhibition of DNA synthesis [21] | |
| 0.575 | chloroquinoxaline sulfonamide | DNA damage [21] | |
| 0.566 | thalicarpine | Inhibition of DNA, RNA, and protein synthesis [24] | |
| 0.543 | flavoneacetic acid | Change the permeability of the tumor vasculature [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sereti, E.; Tsimplouli, C.; Kalaitsidou, E.; Sakellaridis, N.; Dimas, K. Study of the Relationship between Sigma Receptor Expression Levels and Some Common Sigma Ligand Activity in Cancer Using Human Cancer Cell Lines of the NCI-60 Cell Line Panel. Biomedicines 2021, 9, 38. https://doi.org/10.3390/biomedicines9010038
Sereti E, Tsimplouli C, Kalaitsidou E, Sakellaridis N, Dimas K. Study of the Relationship between Sigma Receptor Expression Levels and Some Common Sigma Ligand Activity in Cancer Using Human Cancer Cell Lines of the NCI-60 Cell Line Panel. Biomedicines. 2021; 9(1):38. https://doi.org/10.3390/biomedicines9010038
Chicago/Turabian StyleSereti, Evangelia, Chrisiida Tsimplouli, Elisavet Kalaitsidou, Nikos Sakellaridis, and Konstantinos Dimas. 2021. "Study of the Relationship between Sigma Receptor Expression Levels and Some Common Sigma Ligand Activity in Cancer Using Human Cancer Cell Lines of the NCI-60 Cell Line Panel" Biomedicines 9, no. 1: 38. https://doi.org/10.3390/biomedicines9010038