Abstract
Early Cambrian black shales on the Yangtze Platform host a regionally distributed highly metalliferous sulfide-rich carbonaceous unit which has been the subject of extensive debate. This marker unit, with a few centimeters or tens of centimeters in thickness, displays extreme enrichment in Mo and Ni (wt% range), and in a broad spectrum of other metals such as As, Au, PGE, Re, Cu, Zn, Cd, Ag, Sb, Se, Tl, and Hg, and occurs discontinuously along the western passive margin of the Yangtze Platform. It grades laterally in stratigraphically equivalent meter-thick vanadium-rich shale and tens-of-meter-thick sapropelite (combustible shale). New Cu and Zn isotope data, combined with existing Cd, Cr, Ni, Mo, Hg, and Se isotope and other chemical data, allow to attempt an integrated view on the formation of this intriguing unit of hyper-enriched metalliferous black shale. The authigenic Cu enrichment in the 1000-ppm range has produced no or little Cu isotope fractionation (0.03 ± 0.26 ‰ δ65Cu) beyond the lithogenic background. Heavy zinc and cadmium isotope enrichment in the sulfidic samples (1.11 ± 0.18 ‰ δ66Zn, 0.31 ± 0.10 ‰ δ114Cd) is controlled by sulfide fractionation and contrasts with V-rich and barren shale (0.60 ± 0.18 ‰ δ66Zn, 0.00 ± 0.14 ‰ δ114Cd). The distinctly negative Ni isotope composition of the metalliferous unit (−0.84 ± 0.05 ‰ δ60Ni) with Ni in the percent range has been interpreted as due to hydrothermal activity related to the leaching of mafic rocks and their sulfides. Sorption processes (Fe-oxyhydroxides) and redox cycling in the water column and the bottom sediment with microbial activity could be an alternative interpretation. The extreme metal enrichment can be understood as due to a process chain, from high biological productivity in the oxic photic zone to sulfate reduction in the deeper sulfidic water column and upper sediment layer. Key to the metal enrichment seems to be extremely low clastic sedimentation and advanced carbon destruction by anaerobic oxidation. Hydrothermal input of basinal brines along the rifted margin of the Yangtze Platform was likely a part of this scenario.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Advances in analytical stable isotope geochemistry over the last 20 years allow the measurement of small differences in isotope ratios for an ever-increasing spectrum of elements. Such non-traditional stable isotope data enable the fingerprinting of the bio-/geochemical processes behind isotope fractionations observed and have become essential for the understanding of geochemical cycles at the global to local scale, including ore deposits (Teng et al. 2017).
We, here, present new Cu and Zn isotope data, combined with previously published Cd, Cr, Ni, Mo, Hg, and Se isotope data to elucidate the much-debated origin of a metal-rich ore horizon in the Early Cambrian black shale sequence of the Niutitang Formation on the Yangtze Platform, South China. This thin discontinuous unit, mostly only a few cm thick, of highly metalliferous phosphate-rich black shale with >10 wt% S and >10 wt% Corg is spectacularly enriched in Ni and Mo (in the wt% range), and in a broad spectrum of other metals such as As, Au, Ag, platinum-group elements (PGE), Re, Cu, Zn, Cd, Sb, Se, Tl, and Hg. This unit is irregularly distributed over more than 1000 km along the rifted margin of the Yangtze Platform, with lateral stratigraphic equivalents of non-sulfidic vanadium-rich shale (~0.5 % V), sapropelic alginite (combustible shale with 15–25 wt% Corg) and stratiform barite, which all were, or are, mined (Fig. 1).
The variety of Early Cambrian stratiform ore deposits on the margin of the Yangtze platform within its Early Cambrian geotectonic and paleogeographic setting (from Frei et al. 2020, based on Wang et al. 1985; Jiang et al. 2011; Zhao and Cawood 2012). The area between the Yangtze carbonate platform (very light blue) and the deep-sea basin (blue) is mapped as “starved marginal shallow Yangtze sea” or “protected basin” (Wang et al. 1985) or as “shelf lagoon” (Jiang et al. 2011) and shown in light blue. This area has a broad spectrum of paleo-environments with deposition of black shale, phosphorite, sapropelite, V and Mo-Ni shale, stratiform barite, and very strong lateral changes in stratigraphic thickness, including a chain of paleoislands, the “Jiagnan islands” (grey shaded; only schematically shown). The Early Cambrian is characterized by a marine transgression on the Late Neoproterozoic (Ediacaran) carbonate platform with a locally heavily condensed stratigraphic succession of mainly black shales (Wallis 2007). Note that a similar situation also exists at the northern margin of the Yangtze platform, in the southern Qinling Mountains, although less well understood due to tectonic overprint during Paleo- and Mesozoic collisional events of the South China and North China blocks
The metal spectrum enriched is known from Corg-rich sediments, however, at much lower tenors (Vine and Tourtelot 1970; Ketris and Yudovich 2009; Granitto et al. 2017). There is a broad consensus that metal enrichment is largely of synsedimentary nature, but the metal source is the subject of extended debate. The spectrum of interpretation is between a sedimentary-exhalative model, i.e., metal supply from submarine hydrothermal activity (Coveney and Chen 1991; Coveney et al. 1994; Lott et al. 1999; Steiner et al. 2001; Emsbo et al. 2005; Jiang et al. 2007; Pašava et al. 2008; Wen and Carignan 2011, Och et al. 2013; Jia et al. 2018; Han et al. 2020; Fu et al. 2021) and a seawater model, i.e., metal supply from average seawater under anoxic/euxinic conditions (Mao et al. 2002; Lehmann et al. 2007, 2016; Xu et al. 2012, 2013; Yin et al. 2017; Pagès et al. 2018). A mixed seawater (Mo) and hydrothermal (Ni) origin is advocated by Orberger et al. (2007) and Pašava et al. (2019).
In fact, hydrothermal activity is documented from many MVT-style lead-zinc deposits in the Yangtze carbonate platform which could be related to the downward flux of dense saline brines across the rifted platform margin (Zhou et al. 2014; Zhu et al. 2020). But basinal brines have a specific base-metal signature which is different to the metal signature of the metalliferous black shale unit, which has surprisingly concordant seawater-like REE-Y and PGE-Re-Au patterns (Mao et al. 2002; Lehmann et al. 2003, 2007; Xu et al 2013; Frei et al. 2021).
Geological setting
The Yangtze Block or Yangtze Platform in the Ediacaran/Early Cambrian is a near-equatorial peneplained fragment of the northern margin of East Gondwana, with a then west-facing semi-restricted and sediment-starved passive continental margin (Scotese 2003; Xu et al. 2013; Yao et al. 2014). The metamorphic basement of the Yangtze Platform consists of a poorly exposed Archean nucleus (Zheng et al. 2006) overlain by Paleo- to Early Neoproterozoic slightly metamorphic igneous and sedimentary rocks, including rift- or plume-related komatiitic basalts and ultramafic intrusions (Mao and Du 2002; Wang et al. 2007; Zhou et al. 2017; Huang and Wang 2019). The Late Neoproterozoic/Ediacaran sedimentary record (Doushantuo Formation: 635-551 Ma; Dengying Formation: 551–541 Ma), with up to several hundred m in thickness, indicates several transgressive-regressive events with dominantly shallow-water carbonate and carbonaceous shale sedimentation and locally phosphorite and evaporite deposits (Vernhet et al. 2006; McFadden et al. 2008). The rifted margin displays features of mass-wasting events at all scales (olistostromes) (Vernhet et al. 2006). The Ediacaran to Cambrian boundary is marked by up to several m-thick phosphorite beds and phosphorite nodules in the lowermost black shale of the Niutitang Formation on the karstic surface of the Neoproterozoic carbonate platform of the Dengying and the underlying Doushantuo formations. The phosphorite formed from upwelling nutrient-rich oxic or suboxic water due to shelf subsidence (Zhang et al. 2016). The Niutitang Formation has a very variable thickness from 30 to 75 m in the Huangjiawan area (Xu 2011) to 675 m in an intrashelf basin about 200 km NW of Zunyi (Changye-1 drillhole; Lin et al. 2016; Wang et al. 2018). The rock sequence is moderately deformed by large-scale folds and doming, related to collisional events of the Yangtze Platform and the North China block in the Silurian and Early Triassic (Hsü and Chen 1999). Regional vitrinite reflectance data indicate a high thermal maturity of the Niutitang Formation, corresponding to a temperature of >200°C (Ye et al. 2017; Li et al. 2018; Zhang et al. 2020).
The sulfide-rich unit (about 30 vol% pyrite plus several vol% MoSC phase, millerite/gersdorffite, sphalerite in pyrobitumen-silica-apatite matrix) with extreme metal enrichment is a few m above the disconformable erosional contact to the dolostone of the Ediacaran Dengying Formation, and is continuous over several-km strike length with a thickness of a few up to 60 cm in the Zunyi district, around the Huangjiawan mine (Mao et al. 2002). It grades into vanadium-rich shale over a lateral distance of about 20 km in the Sancha district (Xu et al. 2013; Xu and Mao 2021). The unit consists of thinly laminated sulfide layers (cm thick) and intraformational microbreccia/conglomerate with pyrobitumen and mm-sized clasts and rounded pebbles of sulfidized and phosphatized organic debris with textural relic features of algal mats (Fig. 2). The laminated sulfide layers display cracks perpendicular to the bedding (segmentation); they consist of a repetition of semi-amorphous MoSC phase, pyrite, and organic matter (μm scale). The MoSC-mixed layer phase has the approximate composition of (Mo, Fe, Ni)3(S, As)6C10 (Kao et al. 2001; Orberger et al. 2007). Diagenetic veinlets in all clasts have Ni sulfides (gersdorffite and millerite), sphalerite and minor barite and carbonate. The ore petrography has been extensively documented (Coveney and Chen 1991; Murowchick et al. 1994; Mao et al. 2002; Kříbek et al. 2007; Orberger et al. 2007; Xu et al. 2012, 2013; Han et al. 2020). The textural features have been interpreted as synsedimentary debris flow with clasts of organic material and sulfides in a pyrobitumen-sulfide ± clay matrix (Steiner et al. 2001) and as reworked subaquatic phosphatic hardground (Kříbek et al. 2007).
Typical sample from the Huangjiawan mine with various textural aspects of the sulfide-rich ore unit, from bottom to top: Mottled texture of pyrite aggregates (yellowish white) in fluorapatite matrix (grey) and pyrobitumen clasts (black); mm- thick finely laminated MoSC phase, pyrite and pyrobitumen (dark to light grey); pebbles of MoSC phase (medium grey) and pyrite (white) in pyrobitumen (black)
Bulk organic matter has low H/C atomic ratios (H/Cat ~0.4), both in the metalliferous and barren black shale, which indicate a high degree of thermal maturation (Kříbek et al. 2007). The reflectance corresponds to semianthracite and anthracite, and indicates temperatures characteristic of low-grade metamorphism (prehnite-pumpellyite metamorphic zone, 200–300°C (Kříbek et al. 2007; Orberger et al. 2007). The same temperature interval is also indicated for the late-stage Ni sulfide assemblage of gersdorffite-millerite-polydymite (Belkin and Luo 2008). The organic matter represents remnants of in situ microbially reworked organic matter of cyanobacteria/algal type, and oil-derived material (migrabitumen) (Kříbek et al. 2007). Pyrite nodules with organic matter remains and apatite (francolite: fluorapatite) have δ34S values which range from as low as −26 ‰ to as high as 22 ‰, with repeated cycles at the sub-mm scale, and covering the extremes of δ34S values within a single cycle (Murowchick et al. 1994).
The metal-rich unit is strongly enriched in Re and common Os, at about three to four orders of magnitude higher than bulk continental crust and one to two orders of magnitude higher than the black shale sequence (Horan et al. 1994; Mao et al. 2002; Xu et al. 2011; Lehmann et al. 2016). The Re-Os isochron age is 521 ± 5 Ma (Xu et al. 2011), obtained on samples from three localities, including the Zunyi and Sancha mining districts, several 100 km apart. This age agrees with its biostratigraphic Tommotian age, and the initial 187Os/188Os of 0.8 is similar to the black shale host sequence and to contemporaneous seawater (Jiang et al. 2007). Zircons from a cm-thick layer of volcanic tuff in the vanadium-rich shale at the Sansu/Bagong vanadium deposit, eastern Guizhou, gave a U-Pb age of 521 ± 1 Ma (Wu et al. 2021).
A particular feature of the Early Cambrian black shales are calcite and barite concretions, as well as stratiform barite lenses. Barite concretions with very high δ34S at 68 ± 5 ‰ indicate diagenetic barite formation in the final stages of sulfate reduction (Goldberg et al. 2006), similar to the interpretation of Early Cambrian stratiform barite deposits on the northern margin of the Yangtze Platform (Xu et al. 2016). Žák et al. (2003) report Sr isotope data on barite from the massive barite orebody at Shang Gongtang, Guizhou, which are similar to coeval seawater, while accessory barite from the polymetallic sulfide unit at Huangjiawan is more radiogenic which can be interpreted as of diagenetic or hydrothermal origin (Žák et al. 2003). Phosphorite samples from Huangjiawan indicate wide variability in Sr isotope composition (Decrée et al. 2021), including some data points distinctly below Lower Cambrian (Tommotian) seawater at 87Sr/86Sr ~0.7082 (Li et al. 20132013).
There are many stratiform barite deposits along the Lower Cambrian margin of the Yangtze Craton, often together with chert, which have been interpreted as of SEDEX-type, i.e., related to hydrothermal venting (Wang and Li 1991; Emsbo et al. 2005; Pašava et al. 2008; Han et al. 2015, 2020), although other explanations such as methane seeps and anaerobic oxidation of methane have been proposed (Goldberg et al. 2006; Tatzel et al. 2015; Xu et al. 2016; Gao et al. 2017).
The shale sequence has a depleted δ13Corg composition at about -33 ‰ and similarly depleted carbonate (occasionally as flat, concentric carbonate concretions up to 1 m in diameter) which suggests anaerobic recycling of sedimentary organic matter and thermal degradation (Goldberg et al. 2006, 2007; Guo et al. 2007; Dong et al. 2008). The entire Niutitang Formation has a high shale gas potential and is currently under exploration for unconventional hydrocarbons (Lin et al. 2016; Li et al. 2018; Wang et al. 2018).
It is interesting to note that the northern margin of the Yangtze platform (southern Qinling Mountains) also hosts Early Cambrian V-rich black shale, up to 30 m thick in the Qianjiaping mine, currently the largest V mine in China with 93 Mt @ 0.84 % V2O5 (Golden Share Mining Corp 2016), plus many stratiform barite deposits. Wang and Li (1991) interpreted these Ba-deposits as SEDEX-type, related to submarine hot spring activity during late-stage volcanism, but with their S-O-C isotope signature from the marine depositional environment (seawater). Xu et al. (2016) explained them as largely seawater derived with diagenetic enrichment by cold seeps in relationship to anaerobic oxidation of organic matter.
Samples and analytical methods
The bulk-ore and bulk-rock samples studied are from the Zunyi (Huangjiawan mine, Guizhou province) and Sancha mining districts (Sancha and Xiongjiata, about 25 km southwest of Sancha, Hunan province). They are from the sample collections in Clausthal and Prague, respectively, and have been previously described, including trace elements (Lehmann et al. 2007, 2016; Pašava et al. 2008, 2019; Frei et al. 2020, 2021). These previous studies also included Cd, Cr, and Mo isotope data on the samples from Clausthal, and Ni isotope data on the samples from Prague.
The samples of the sulfide-rich black shale unit are grab samples from underground exposures at the Huangjiawan and Sancha mines; the V-rich samples are from surface exposures (ZH-3 and -4) in the Zunyi district and from a road cut near Zhangjiajie (XT-2 and -5) in the Sancha district, including the shallow exploration drillcore ZK 3001 in the same area (ZK 3001-2 at 4 m depth, where also the barren shale sample is from (ZK 3001-8 at 29 m depth) (Xu 2011). The eight samples (1–2 kg) from the Huangjiawan section, labeled as CHI in ESM Table 1, cover about 3 m of stratigraphic thickness and are from surface exposures (Pašava et al. 2008). This cross-section reaches from the basal phosphorite unit to the black shale overlying the sulfide-rich marker unit. The full chemical dataset is shown in ESM Table 1. Part of the Cd isotope data was already presented in Frei et al. (2020). The Cr and Mo isotope data are from Lehmann et al. (2016), and the Ni isotope data are from Pašava et al. (2019).
Sample preparation for Zn and Cu isotope analysis
Sample preparation for isotope measurements was done at the Czech Geological Survey. Measured volumes of each sample were evaporated in Teflon containers to concentrate 1-2 μg of Zn and >200 ng of Cu (cf. Borrok et al. 2008). The evaporated material from each sample was then dissolved in 0.5 mL of 6 M HCl, and a few drops of H2O2 were added for total oxidation of Cu. Copper and zinc were separated from the matrix by elution chromatography using anion exchange resin AG-1-X8 (200–400 mesh, Bio-Rad, USA). The method used in this study was designed based on the modification of the procedure by Chapman et al. (2006) and is similar to that described in Novák et al. (2016). Resin in 10 mL PE column was washed with 10 mL of 2 % HNO3 followed by the addition of Milli-Q water to reach neutral pH. Afterwards, the resin was pre-conditioned with 6 mL of 6 M HCl, and the sample was loaded. After the entire sample volume was loaded on the resin, cations of the matrix were eluted by 4 mL of 6 M HCl. In order to collect the Cu fraction, 10 mL of 5 M HCl was used. Subsequently, to elute Fe, 10 mL of 1M HCl was added into the column. Zinc fraction was collected using 10 mL of 0.5 M HNO3. After chemical separation, the Cu and Zn fractions were dried down twice with the addition of 100 μL of concentrated HNO3 to remove chlorides and remnants of the organics. Samples were re-dissolved with 5 mL of 2 % HNO3 for isotope analysis.
Cu and Zn isotope measurements
Isotope analysis was performed on a double-focusing multicollector inductively coupled plasma mass spectrometer (MC ICP-MS) Neptune (Thermo) equipped with nine Faraday detectors at the Czech Geological Survey. For Cu isotope analysis, samples were introduced into the Ar plasma via a cyclonic glass spray chamber, whereas for Zn measurement, the desolvating nebulizer Aridus II (Cetac, USA) was used.
During Cu isotope analysis, the instrument was operated in low-resolution mode (M/ΔM(95%) = 300) with masses 60 and 62 (for Ni), and 63 and 65 (for Cu) simultaneously measured. For Cu concentration of 100 μg/L, an intensity of 6 V was routinely observed at 63Cu. All samples were spiked by the Ni standard NIST 986 for instrumental mass bias correction, and the method of standard sample bracketing (ERM-AE 633 standard for Cu) was applied to obtain δ65Cu.
In the case of Zn, isotope masses 64, 66, 67, 68, and 70 were simultaneously detected in a medium-resolution mode (M/ΔM(95%) = 4000). The Zn standard SRM NIST 683 was used, and the signal intensity of 5 V was routinely measured at 66Zn for a concentration of 100 μg/L. A 67Zn – 70Zn double spike was utilized for mass bias correction. The description of the double-spike preparation is given in detail in Voldřichová et al. (2014). Isobaric interferences on the mass 64 (between 64Ni and 64Zn) were controlled by monitoring the signal at 62Ni. The signal from this isotope was insignificant (<10 mV) during all measurements.
During both Cu and Zn analyses, the instrument was washed out after every single sample or standard measurement. The utilized analytical blank contained 2 % HNO3, and appropriate standards were run after every four samples. Measurements were carried out within one block of 40 cycles, and signals for each cycle were integrated for 4 s. All samples were analyzed in duplicates, from which the analytical errors were calculated. The accuracy of the Cu and Zn isotope measurements was controlled by replicate measurement of the BHVO-2 and SCo-1 standards; results were in good agreement with δ65Cu values published by various authors in the GeoRem database (Jochum et al. 2005). Accuracy and reproducibility of δ66Zn values were assessed by replicate analyses of the BHVO-2 (0.27 ± 0.06 ‰), PCC-1 (0.24 ± 0.07 ‰), and GSJ JP-1 (0.16 ± 0.07 ‰) standards. Our results agree well with previously published values (Makishima and Nakamura 2013; Sossi et al. 2015). The average measurement uncertainty for δ65Cu was ≤0.08 ‰ (2 SD) and that for δ66Zn was ≤0.06 ‰ (2 SD).
Copper isotopic ratios are reported against the AE633 standard (equal to NIST SRM976 -0.01 ‰) and those of Zn against the JMC Lyon standard (Zn JMC 3-0749L), respectively:
Results
The results of the isotope analyses are shown in ESM Table 1 together with a wide range of other chemical data (major elements, trace elements, including REE and PGE data), and previously published Cr, Cd, Mo, and Ni isotope data on the same sample set. The bulk Cu and Zn concentrations were transformed into authigenous Cu and Zn concentrations, by subtraction of the lithogenic (detrital) element content derived from the composition of upper continental crust normalized to Al content (Taylor and McLennan 1985), as detailed in Lehmann et al. (2016), for example. The authigenous Cu and Zn concentrations are nearly equal to bulk rock in the sulfide-rich samples and slightly lower in the non-sulfidic samples. The difference is such that the Cu and Zn isotope plots are not significantly affected by this correction.
The δ65Cu data have a spread of about 1.6 ‰ (1.1 ‰, if drillcore sample ZK 3001-8 of barren black shale is omitted), which is relatively narrow given the large spread of about 25 ‰ observed in natural samples (Mathur et al. 2009). The broad global spread is due to the transition between various oxidation states in which Cu can exist (0, 1+, 2+), while phase changes under constant oxidation state produce only minor isotope fractionation, as observed in our samples.
The δ66Zn data range from 0.41 to 1.45 ‰ and display a distinct clustering of isotopically heavy Zn in the sulfide-rich samples. The δ114Cd data with a range of −0.18 to 0.41 ‰ mirror this pattern, with lighter Cd in the V-rich and barren shale samples which have around 0 to negative δ114Cd values (Frei et al. 2020, 2021).
Discussion
Copper
The Cu isotope data show scatter around the average spread of global igneous and clastic rocks at 0.08 ± 0.17 ‰ δ65Cu (n = 287; 1s) (Moynier et al. 2017) (Fig. 3), and do not correlate with the Zn isotope data. They, however, show a significant correlation with δ60Ni in the set of samples from the Zunyi mining district described in Pašava et al. (2019). The limited spread in the data could be due to sorption processes (both abiotic and biotic), which tend to enrich isotopically heavy Cu, and to microbial metabolic uptake which tends to enrich isotopically light Cu (Moynier et al. 2017). The low-Cu samples tend to have isotopically lighter Cu, probably due to fixation by organic material where kinetic fractionation leads to preferential uptake of isotopically light Cu into phytoplankton cells in the photic zone.
Plot of δ65Cu versus Cu concentration (authigenous). The samples have a relatively small variation range around the global lithogenic value of 0.08 ± 0.17 ‰ δ65Cu (1s), with the high-Cu samples at 0.2± 0.1 ‰ δ65Cu, similar to the very narrow bioauthigenic range of euxinic Black Sea and Cariaco Trench sediments (Little et al. 2017), but also within the much larger range of massive sulfide deposits (−1 to +3 ‰ δ65Cu; Ikehata et al. 2011). Error bars are 2s
The exceptional heavy Cu isotope composition with 1.04 ‰ δ65Cu of the barren shale sample from Sancha with only 63 ppm Cu and elevated Al2O3 content could be understood as due to sorption of aqueous Cu on clay minerals. Experimental data from Pokrovsky et al. (2008) give a Δ65Cusorbed-solution = 1.0 ± 0.2 ‰ for gibbsite. However, and perhaps more likely, the elevated δ65Cu value could also derive from slight weathering, where oxidative leaching of Cu1+ sulfide would lead to remineralization of Cu2+ phases with 65Cu enrichment (Mathur et al. 2005) as documented for Paleozoic black shales (Mathur et al. 2012; Lv et al. 2016).
The next-heavy sample with δ65Cu of 0.54 ‰ has a much higher Cu concentration (1180 ppm Cu, Sancha), much lower Al2O3, and is from the Ni-Mo sulfide unit. This value is in the δ65Cu range of average modern seawater of 0.5 (near-surface) to 0.6 ‰ (near-bottom) (Takano et al. 2014), or 0.6 to 0.9 ‰ (Vance et al. 2008), and could be due to near-quantitative scavenging into sulfide. However, other samples with elevated Cu content are in the range of 0.1–0.3 ‰ δ65Cu and suggest no fractionation at all or a more complex sorption-resorption mechanism.
There is a cryptic positive correlation between δ65Cu and S content (Fig. 4), omitting the anomalously elevated δ65Cu value of the barren shale sample. The high-S (and high-Cu) samples have slightly positive δ65Cu values in the range of 0 to 0.3 ‰. This is within the range observed for volcanogenic massive sulfide deposits which have −1 to +3 ‰ δ65Cu (Ikehata et al. 2011) and modern altered oceanic crust with −0.1 to +0.3 ‰ δ65Cu (Huang et al. 2016) but also corresponds to observations from modern marine sediments deposited under euxinic conditions. Samples from euxinic depositional intervals from the Black Sea and Cariaco trench display a bioauthigenic Cu enrichment (i.e., from seawater) with a small positive δ65Cu signature up to 0.3 ‰, while oxic depositional intervals have lithogenic δ65Cu composition close to 0 ‰ (Little et al. 2017). The bioauthigenic signature is explained as resulting from organic complexing of Cu, the dominant form of dissolved Cu in all aquatic environments, where strong organic ligands preferentially complex the heavy Cu isotope (Little et al. 2017).
We conclude that the Cu isotope signature does not allow a clear constraint on the Cu source, but identifies very limited aqueous fractionation beyond the global lithogenic δ65Cu range (Moynier et al. 2017), different to other metal-rich black shales with a larger range in δ65Cu values, such as the Neoproterozoic-Cambrian black shales in the Bohemian Massif (Ackerman et al. 2019), but similar to modern euxinic sediments of the Black Sea and Cariaco Trench (Little et al. 2017).
Zinc
The Zn isotope data range from about 0.4 to 1.4 ‰ δ66Zn (Fig. 5). The low-Zn samples with <1000 ppm Zn have a mean of 0.65 ± 0.18 ‰ δ66Zn which is higher than average shale which has a lithogenic δ66Zn value of about 0.30 ± 0.07 ‰ (n=77; 1s), similar to Bulk Silicate Earth (Moynier et al. 2017). However, it is close to global present-day deep ocean seawater with 0.51 ± 0.14 ‰ (Vance et al. 2016). Sorption to organic matter fractionates many isotopes, including Zn, towards heavier isotopic composition in the organic matter, and may produce a shift in δ66Zn values of about +0.28 ‰ (Jouvin et al. 2009). Similarly, sorption to clay minerals can also produce a positive shift of δ66Zn values up to 0.5 ‰ (Guinoiseau et al. 2016). Both processes could explain the isotopic features of the low-Zn samples. Biologically incorporated zinc must be less important because Zn in living cells is fractionated towards lighter isotopic composition (John et al. 2007). Hydrothermal mid-ocean vent fluids have a relatively light Zn isotopic composition of about +0.24 ‰ δ66Zn (Little et al. 2014) or much less, as in the TAG field where the hydrothermal end-member is approximated by a δ66Zn value of -0.5 ‰ (Conway and John 2014). The hydrothermal plume at the East Pacific Rise has an end-member composition of 0.24 ‰ δ66Zn, which is similar to crustal values and to other sources of Zn to the oceans (John et al. 2018). These findings are inconsistent with the data observed in our sample set.
Plot of δ66Zn versus Zn concentration (authigenous). The position of the low-Zn samples with <1000 ppm Zn can be explained by the sorption of Zn to organic matter. The sulfidic high-Zn samples display a shift towards isotopically heavier Zn compared to the global lithogenic δ66Zn value of about 0.30 ± 0.07 ‰ (1s), while the non-sulfidic V-rich shale samples, mostly also high in Zn, are largely unfractionated. The sulfidic high-Zn samples from the Ni-Mo shale likely relate to sulfide precipitation in the euxinic water column, with Zn fractionation either in the water column, analogous to the Black Sea, or to seafloor venting of fractionated basinal brines. Error bars are 2s
However, individual fluid samples from active hydrothermal vents (John et al. 2008) and low-temperature hydrothermal ore deposits (Wilkinson et al. 2005) span a very large range in Zn isotopic compositions from −0.17 to 1.33 ‰ δ66Zn, and the large spread is probably due to kinetic fractionation processes involving the preferential incorporation of isotopically light Zn into sphalerite along the fluid path. Therefore, given the lack of knowledge of the paleohydrologic situation, hydrothermal input in the sense of a model of venting of oxic basinal brines (Pašava et al. 2008) cannot be excluded.
The high-Zn data appear to have an erratic δ66Zn distribution with a large spread (Fig. 6). However, when grouped according to sulfur content, there is a consistent bimodal pattern: (i) Samples with low sulfur content of <5 wt% S, and (ii) those with elevated sulfur up to 25 wt% S. The latter group comprises all samples of the Mo-Ni sulfide layer, while the low-S group comprises V-rich shale and shale within less than 1 m below and above the Mo-Ni sulfide layer, including barren shale. The low-S samples plot within a δ66Zn range of 0.18 to 0.73 ‰ which overlaps with global igneous and clastic rocks as well as mid-ocean vent fluids at δ66Zn of 0.30 ± 0.13 ‰ (Moynier et al. 2017). This range can be further refined by separating the V-rich and the barren samples with a relatively narrow δ66Zn range of 0.18 to 0.52 ‰ (n=6), from the samples from the Huangjiawan section immediately above and below the sulfide layer with a δ66Zn range of 0.26 to 0.73 ‰ (n=7). The high-S samples from the Ni-Mo sulfide layer have a δ66Zn range of 0.71 to 1.25 ‰ (n=7). All samples together display two trends in the Zn vs δ66Zn plot of Fig. 5: (i) The vertical trend of the low-S samples indicates no or little Zn isotope fractionation compared to the lithogenic baseline, in spite of significant Zn enrichment in the vanadium shale; (ii) the trend with positive slope relates to significant Zn isotope fractionation which correlates with S and Zn tenors. This positive correlation suggests sulfide control of the Zn isotope signature. There is no correlation with TOC (total organic carbon) content, and the sample of vanadium shale with the highest TOC value of 23.4 wt% has a low δ66Zn value of 0.22 ‰. Nevertheless, the syndepositional TOC value is not known, and degradation and thermal maturation could shift residual organic-rich sediments towards heavier Zn isotope composition because expelled organic fluids would have lighter Zn isotope composition by about 0.4–0.6 ‰ δ66Zn (Dickson et al. 2020).
Plot of sulfur content versus δ66Zn. The sulfidic samples gave a significantly heavier Zn isotope composition than the non-sulfidic samples. Compare to the Black Sea water column, with δ66Zn values of 0.2–0.3 ‰ in shallow water, and 1.0–1.3 ‰ in the water below the redoxcline at 150 m (Vance et al. 2016). Both quantitative removal of seawater Zn from the euxinic water column and sulfide fixation of external Zn from seafloor venting of fractionated oxic basinal brines are possible. Error bars are 2s
The two trends of Zn enrichment with (1) enrichment of isotopically heavy Zn (Mo-Ni shale), and (2) enrichment of isotopically light Zn (V-rich shale) to about the same level of Zn enrichment (Fig. 5) suggest that these patterns did not derive from one process, such as hydrothermal overprint, but are due to two different mechanisms. These could be the marine paleo-environment, with (i) a euxinic water column and change in Zn speciation with corresponding Zn isotope fractionation in the water column related to the metalliferous sulfide-rich unit, and (ii) an anoxic water column with H2S concentration insufficient for a change in speciation and Zn isotope fractionation, related to the non-sulfidic V-rich shale. This aspect will be further explored below. The two different trends also indicate that the samples have probably preserved a largely primary signature, and were not homogenized by later processes.
The metal- and sulfide-rich samples have consistently a heavy Zn isotope signature. Intriguingly, their composition is similar to average deep-sea ferromanganese nodules with δ66Zn of 0.90 ± 0.28 ‰ (n=41) (Maréchal et al. 2000) or 1.04 ± 0.21 % (n=22) (Little et al. 2014). These nodules formed by sorption/desorption processes over a long time, from deep seawater with a relatively constant present-day composition of 0.51 ± 0.14 ‰ δ66Zn (Little et al. 2014; Vance et al. 2016). The same applies to biogenic carbonates which have a δ66Zn value of 0.91 ± 0.24 ‰ (Pichat et al. 2003). The isotopically heavy Zn composition was explained as due to high biological activity which would extract isotopically light Zn and enrich the upper water column in isotopically heavy Zn, which would then settle down in biogenic particles. Given the extreme variability in Zn concentration (about 2 orders of magnitude) and Zn isotope composition (about 2 ‰) in the modern oceans, largely due to the massive drawdown of zinc by phytoplankton in the surface ocean, our data have the recurrent problem that the paleohydrology of the depositional system is not sufficiently understood.
Nevertheless, a comparison to the well-studied Black Sea, a permanently anoxic and restricted basin, can be attempted. Vance et al. (2016) present Zn isotope data over the 2000 m water column. The surface water down to 70 m depth has 0.23 ± 0.05 ‰ δ66Zn, and biological near-surface uptake of Zn seems to involve no significant isotope fractionation. However, there is a dramatic shift at the redoxcline to isotopically heavier dissolved Zn by about 0.9 to 1 ‰, accompanied by a strong decrease in dissolved Zn. This change relates to Zn speciation. The dominant inorganic dissolved species of Zn in the sulfidic zone is ZnS(HS)-, as opposed to the dominant Zn2+ species in the upper oxic zone (Vance et al. 2016). The magnitude of the Zn isotope fractionation between these species is Δ66Zn (Zn2+-ZnS(HS)− = +0.73 ‰ at 25°C (Fujii et al. 2011). The water column at the redoxcline is likely close to sphalerite equilibrium, and the near-quantitative removal of Zn (and likely other redox-dependent metals) could then lead to a haze of organic material and sulfide micro-particles in the deeper water column with δ66Zn at about 1 ‰. Such heavy Zn is not seen in the sediments of the Black Sea, which have only a few 10s of ppm Zn, and which are dominated by clastic sedimentation with a high depositional rate.
A recent study by Fan et al. (2020) provided a large Zn isotope dataset on the Early Cambrian black shales and their metalliferous sulfide unit. The samples came from the same two areas studied here, i.e. Zunyi (Huangjiawan mine) and Sancha (Zhangjiajie), and the data confirm the bimodal distribution, i.e., black shales with low Zn content are in the δ66Zn range of 0.51 to 0.89 ‰, whereas sulfide-rich samples with >1000 ppm Zn are in the δ66Zn range of 0.87 to 1.41 ‰. In addition, Fan et al. (2020) also studied the Nayong prospect, 150 km SE of Zunyi. This area has black shales with a Zn isotope signature similar to the black shales at the other two localities. However, the metalliferous sulfide unit at Nayong is exceptionally Zn-rich with the four samples studied at 10.5 ± 3.7 wt% Zn and 0.57 ± 0.05 ‰ δ66Zn, and sphalerite in these samples on veinlets. Fan et al. (2020) suggested a hydrothermal origin for the Zn enrichment in the Nayong prospect, which would correspond to the occurrence of MVT-style Pb-Zn mineralization in the underlying Dayong dolostone sequence within the same area, and to a similar δ66Zn signature in many hydrothermal systems (Wilkinson et al. 2005; Zhou et al. 2014). However, the authors also related the isotopically heavy Zn signature in the sulfide-rich samples from Zunyi and Sancha with one order of magnitude lower Zn content to a hydrothermal origin, by proposing a Rayleigh fractionation model for basinal brines over a plumbing system several 100 km large along the NE-trending margin of the Yangtze Platform, i.e. increasing δ66Zn from Nayong (0.57 ± 0.05 ‰; n=4) to Zunyi (1.01 ± 0.12 ‰; n=3) to Sancha (1.35 ± 0.10 ‰; n=4), over a distance of about 600 km, and in accord with a decreasing Zn content in the sulfide-rich unit towards NE. Our own elevated δ66Zn data for Zunyi are 1.14 ± 0.18 ‰ (n=6), and for Sancha 0.98 (one sample only). We cannot confirm a regional increase in Zn content in the sulfide-rich unit, which indeed is elevated in the Maluhe area (our own data: 2.28 ± 0.66 wt% Zn; n=6), but is at a lower but very similar level in the Huangjiawan, Sancha and Cili deposits (70 km NE of Sancha) with 0.38 ± 0.14 wt% Zn; n=11) (Xu 2011). The sulfide-rich unit is very thin in the Maluhe area and grades into V-rich shale towards SW (Xu and Mao 2021), while in the Cili area this unit grades NE-ward into combustible shale (“stone coal”).
Rayleigh fractionation has been demonstrated for several Pb-Zn deposits, with isotopically light Zn at the initial deposition front, and isotopically heavier Zn at the final deposition front (Wilkinson et al. 2005; Zhou et al. 2014). The study by Kelley et al. (2009) did show such a cryptic trend from 0.0 to 0.2 ‰ δ66Zn within the Lower Carboniferous shale-hosted massive sulfide Red Dog deposit, with breccia samples from the Anarraaq prospect 13 km away at 0.4–0.6 ‰ δ66Zn. However, the genetic relationship between these Zn-Pb-Ag deposits is unclear, and the data range at Anarraaq corresponds to the global range of marine black shale, as also observed in the Early Cambrian black shale sequence in South China.
Other isotopes
Cadmium
Cadmium isotope data on the same sample set, reported in Frei et al. (2020, 2021) and in ESM Table 1 can provide additional information and confirm the interpretation of the Zn isotope data. Zinc and cadmium are correlated, both in elemental and isotope plots (Fig. 7), because Cd can substitute for Zn and both elements behave very similarly. The data for the Huangjiawan sulfide- and metal-rich unit have a narrow range with a mean value of 0.35 ± 0.03 ‰ δ114CdNIST3108 (n = 5), while the other 7 samples cover a range of 0.09 to −0.19 ‰ δ114CdNIST3108. The isotopically lightest Cd composition around −0.2 ‰ is in the V-rich shale from Huangjiawan (n = 2), while the samples from Sancha display a δ114Cd value around 0.1 ‰ (n = 5). These data are similar to the results by Hohl et al. (2019). The isotopically lighter Cd in the non-sulfidic rocks could be related to biological Cd fixation because phytoplankton preferentially takes up light Cd isotopes with a fractionation factor Δ114Cd of up to 0.34 ± 0.14 ‰ (Lacan et al. 2006). The isotopically heavier Cd could relate to sulfide precipitation in the water column, in a similar way as suggested for Zn. The modern deep ocean water has a Cd isotope composition of 0.35 ± 0.12 ‰ δ114Cd with increasing values towards the photic zone with up to 0.9 ‰ δ114Cd (Sieber et al. 2019; Bryan et al. 2021). As for the reasoning for the Zn isotopes, the elevated δ114Cd composition of the sulfide- and metal-rich unit likely represents quantitative removal of Cd, possibly as rain-out at and below the redoxcline (Frei et al. 2020, 2021). A similar conclusion was put forward by Sweere et al. (2020) for black shales from the Upper Cretaceous Maverick Basin (TX, USA). These authors attribute high δ114Cd values to near-quantitative removal as sulfide particles from seawater in euxinic environments and lower δ114Cd values in non-euxinic parts of the basin to partly remineralized organic material settled from the photic zone.
Plot of δ66Zn versus δ114Cd. The sulfide-rich samples have an isotopically heavy Zn and Cd composition similar to deep water in euxinic basins (Vance et al. 2016; Sieber et al. 2019; Sweere et al. 2020) and could represent the local seawater Cd-Zn isotope composition. V-rich shale and barren shale are less fractionated compared to the global lithogenic Zn and Cd composition of about 0.3 ‰ δ66Zn and about 0.0 ‰ δ114Cd. Error bars are 2s
However, similar to the Zn isotope system, low-T Rayleigh fractionation processes in hydrothermal systems give a similar pattern, with δ114Cd values in between 0 and 0.8 ‰ as observed in MVT deposits in China (Wen et al. 2016). Interestingly, sedimentary-exhalative deposits have δ114Cd values from 0.5 to −0.4 ‰, similar to most modern seafloor deposits, and also have very high Zn/Cd ratios of >200 (Wen et al. 2016).
Nickel
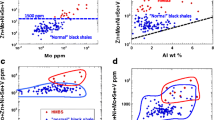
Nickel isotope data on the Huangjiawan section have been published by Pašava et al. (2019) and are compiled in ESM Table 1. The data show a significant variation of 0.9 ‰ in δ60NiSRM986 for the Huangjiawan black shale section, with −0.71 to 0.08 ‰ (n = 6), and the sample from the sulfide-rich unit at −0.84 ‰. This variation is similar to the variation range of 0.9 ‰ for the sulfide-rich unit in the Nick property, Canada, with drill core samples of −0.39 to 0.54 ‰ (n = 5) on the a-few-cm-thick rock unit in 5 drillcores (n = 5) (Pašava et al. 2019). The largest spread was observed in Ediacaran black shales in the Bohemian Massif, Czech Republic (drillcore) which range from −0.46 to 0.62 ‰ δ60Ni (n=13) (Pašava et al. 2019).
The δ60Ni data correlate positively with the δ65Cu data for the sample section at the Huangjiawan mine, with the Mo-Ni-rich ore unit plotting apart (Fig. 8). Such a correlation is also known from sulfides in the Sudbury intrusion (Christoffersen 2017) and was interpreted there as Rayleigh fractionation in a closed magmatic system, with the lowermost δ60Ni data point apart as indicating hydrothermal alteration. By analogy, the black shale data could be related to a relatively closed aqueous system. The basic observation of the correlation plot in Fig. 8 is the fact that there is significant isotope fractionation in a dynamic system.
Plot of δ60Ni versus δ65Cu for the samples of the Huangjiawan section, compared to data from sulfide and rock samples in the magmatic Sudbury system (grey symbols) from Christoffersen (2017). One data point from Sudbury is outside of the plot at −1.69 ‰ δ60Ni and −0.92 ‰ δ65Cu and corresponds to altered mafic rock. Error bars are 2s
The unusually low δ60Ni value of −0.84 ‰ for the Mo-Ni sulfide-rich unit was interpreted by Pašava et al. (2019) as a source signature of alteration of ultramafic rocks and their sulfides because such samples have a light Ni isotope composition (Gueguen et al. 2013; Ratié et al. 2015, 2018). The leaching of such lithologies could result in the enrichment of hydrothermal brines in Ni, Cu, Fe, PGE, and other metals. Ultramafic rocks are part of the Meso-Neoproterozoic basement of the Yangtze Platform, but exposures of such rocks are known only about 100 km away from the Huangjiawan and Sancha mining districts. Note, however, that mass balance requires that the isotopically light Ni composition of leached ultramafic rocks must be compensated by the export of isotopically heavy Ni to the leach solution, as observed in groundwater from ultramafic rocks (Barro Alto complex, Brazil: δ60Ni up to +2.3 ‰, Ratié et al. 2015, 2018) and more generally in river waters (Amazon river: δ60Ni up to +1.4 ‰, Revels et al. 2021).
The continental lithogenic (equal to Bulk Silicate Earth) composition is 0.1 to 0.2 ‰ δ60Ni (Gall et al. 2017; Klaver et al. 2020), compared to global seawater with 1.4 ± 0.2 ‰ δ60Ni, and hydrogenetic deep-sea Fe-Mn nodules and crusts at about 1.6 ‰ (Gueguen et al., 2016). Aqueous oxic-sulfide fractionation Δ60Ni (Ni2+ - NiS(HS)− has been estimated at 0.7 ‰ at 25°C (Fujii et al. 2014). This explains the generally positive δ60Ni composition of recent black shales, such as those in the Black Sea with δ60Ni values of 0.3 to 0.6 ‰ (Vance et al. 2016). The corresponding negative oceanic sink seems to be Mn-rich deep sea sediments, as found for the eastern Pacific with δ60Ni values of −0.2 to −0.8 ‰ (Little et al. 2020). The elevated δ60Ni values of Fe-Mn crusts are explained by the influence of diagenetic fluids with about 3 ‰ δ60Ni (Little et al. 2020). Strong Ni isotope fractionation on sorption of Ni on Fe- and Mn-oxides is experimentally determined with a δ60Ni value of up to 3 ‰ for Mn oxide phases (Sorensen et al. 2020). This could also explain the negative δ60Ni composition down to −1.5 ‰ δ60Ni of low-Ni (up to 200 ppm) Fe-Mn crusts on the Loihi seamount, an active submarine volcano of the Hawaiian-Emperor seamount chain, with a combined low-T hydrothermal and seawater source of some metals, including Ni (Gueguen et al. 2021).
Nickel displays an unusually large fractionation range compared to other intermediate-mass metal isotope systems, and the Black Sea water column, for instance, has a Ni isotope variation range of about 1.5 ‰ (Vance et al. 2016), possibly much controlled by microbial fractionation processes (Archer et al. 2020). Nickel content and Ni isotopes are biomarkers that can trace specific microorganisms and their metabolic processes (Scherer et al. 1983; Cameron et al. 2009). Nickel is essential for methanogenesis by anaerobic microorganisms, the methanogens, which have a specific Ni-containing co-enzyme (MCA) (Scheller et al. 2010). Different from other microorganisms, methanogens produce a significant Ni isotope fractionation with Δ60Ni (cells - aquatic medium) of −0.44 ± 0.20 to −1.46 ± 0.08 (Cameron et al. 2009). Methanogens have an elevated Ni content of 100 ±47 ppm Ni (40 wt% C), which is a specific marker of these microorganisms (Scherer et al. 1983). A 99 % degradation rate (10 x more than the usual marine decay rate) of the organic components in the bottom sediment then could bring the remaining material to the percent range in Ni, which would be quantitatively fixed due to the sulfidic environment. Support for this scenario comes from recent work on Ni isotopes in authigenic pyrite of the Nantua Formation, South China (equivalent to the Marinoan glaciation) where Zhao et al (2021) observed Ni isotopic compositions with down to −0.5 ‰ δ60Ni, and a negative correlation between Ni content and δ60Ni values, interpreted as due to the degradation of biomass from methanogens with coexisting microbial sulfate reduction and methanogenesis in the sulfidic water column.
Nickel is a strongly particle-reactive element and the Fe-Mn oxyhydroxide particulate shuttle model for Mo isotope fractionation (see below) may also apply to the Ni isotope system. Adsorption of Ni to Fe-Mn oxyhydroxides or organic matter preferentially removes lighter Ni isotopes from solution, with experimentally observed isotopic offsets of ∆60Ni (ferrihydrite-water) of −0.35 ± 0.10 ‰ (Wasylenki et al. 2015) and ∆60Ni (birnessite-water) of −2.8 to −3.4 ‰ (Sorensen et al. 2020). These data correspond to natural Ni isotope fractionation in regoliths above ultramafic rocks (Ratié et al. 2015, 2018). Applied to a redox-stratified water column, Fe-Mn particulates sequester isotopically light Ni from average seawater in the photic zone, aggregate and then sink into anoxic waters, where the reductive dissolution of the particulates occurs, releasing Fe, Mn, and adsorbed ions including Mo, Ni, and V. This “particulate shuttle” enriches the deeper water column in particle-active elements with a fractionated isotope pattern, i.e., light Ni (and Mo) compared to average seawater. Under sulfidic conditions, the isotopically light Ni desorbed from particulates then can become sulfidized and removed to sediments. The shuttling process and concomitant Ni isotope fractionation may also occur below the sediment-water interface. Modern metalliferous sediments in the Guatemala basin are enriched in Ni with a light isotope composition of 0 to −1 ‰ δ60Ni, explained as a result of cycles of Mn-oxide dissolution with the release of sorbed Ni, and resorption on newly precipitating Mn oxide phases within the shallower oxygenated sediment zone (Little et al. 2020).
Chromium
Chromium isotope data on the sample set have been published by Lehmann et al. (2016) and Frei et al. (2020) and are tabulated in ESM Table 1 and shown in Fig. 9. Chromium is particularly enriched in the V shale, where both Cr and V are fixed under suboxic and anoxic bottom water denitrifying conditions. This is different from Mo fixation which requires fully anoxic and euxinic conditions (Piper 1994; Piper and Calvert 2009). The positive Cr isotope range of 0.42 to 1.47 ‰ δ53CrSRM979 is similar to what is observed in the organic-rich mud sediments of the 4- to 14,000-year-old Cariaco basin (Reinhard et al. 2014), and can be interpreted as a signal of oxidative continental weathering from the photic zone of the seawater column, modified by adsorption/reduction processes (Lehmann et al. 2016). The highly positive δ53Cr values of 1.83 ± 0.76 ‰ in the ~0.56 Ga marine black shales from the Bohemian Massif represent additional evidence for a highly oxic surface environment during this time (Ackerman et al. 2019).
Plot of δ66Zn versus δ53Cr. The barren shale sample plots at the global lithogenic composition. The V-rich shale samples are possibly more controlled by biological fractionation (phytoplankton) and sorption processes, while the sulfide-rich Mo-Ni±Zn-rich samples are possibly more controlled by sulfide fractionation (ZnS precipitation). Error bars are 2s
This range is much different from the clastic Cr isotope signature of the barren shale sample with the non-fractionated δ53Cr value of about zero, typical of average silicate rocks (Schoenberg et al. 2008). The data in Fig. 9 display a negative correlation between δ66Zn and δ53Cr, with the sample of barren shale apart, with a lithogenic Zn and Cr isotope composition near bulk silicate Earth. The samples of V-rich shale are highly fractionated with respect to the Cr isotope composition, and less fractionated with respect to the Zn isotope composition. This is opposite to the sulfide-rich Mo-Ni shale samples and can be interpreted as sulfide control in the euxinic environment, while the V-rich shale samples reflect biogenic fractionation from oxic seawater and remineralization of organic matter.
Molybdenum
The molybdenum isotope composition of part of the sample set analyzed here was published by Lehmann et al. (2007), and data on additional samples from the Zunyi and Sancha districts have been published by Xu et al. (2012, 2013). These data define a mean δ98MoSRM3134 value of 1.08 ± 0.10 ‰ (n = 9) for the metalliferous sulfide unit. Given this relative homogeneity and that Mo isotopes can archive seawater composition in case of quantitative Mo removal at elevated H2S concentration (Erickson and Helz 2000; Barling et al. 2001), this value could be the ocean signature at about 521 Ma, the age of the sulfide-rich shale marker unit. The data could suggest that the Early Cambrian oceans had more sulfidic depositional environments than the modern oceans (Lehmann et al. 2007; Wille et al. 2008). This conclusion is also supported by Kurzweil et al. (2015) who interpreted an Early Cambrian seawater composition of δ98Mo = ~1.1 ‰ from Ediacaran black shales in the Bohemian Massif, Czech Republic.
However, the barren and non-sulfidic V-rich black shale sequence with Mo in the ppm range has variable Mo isotope composition from −0.1 to 2.3 ‰ δ98Mo (ESM Table 1). The value of 2.3 ‰ for barren black shale with 21 ppm Mo is intriguingly identical to modern seawater at 2.34 ± 0.10 ‰ δ98Mo (Nägler et al. 2013; Nakagawa et al. 2012), while the lower δ98Mo values may reflect non-quantitative Mo removal and/or fractionation processes due to repeated sorption and adsorption processes on suspended particles in the water column (Goldberg et al. 2009; Scholz et al. 2013). Near-present-day seawater composition was also deduced from Mo isotope data in the uppermost part of the Dengying Formation with up to 2.1 ‰ δ98Mo (Kendall et al. 2015), and from a deep drill hole for shale gas exploration into the Niutitang Formation which gave δ98Mo values of 0.0 to 2.0 ‰ (Yin et al. 2018). The work by Xu et al. (2012) identified δ98Mo values up to 1.7 ‰ in the Niutitang black shales. Therefore, it appears possible that the Early Cambrian seawater was at least episodically not far from the oxidation state of the modern oceans. The δ98Mo value of about 1.1 ‰ for the polymetallic sulfide-rich marker unit could represent a transient episode of regional ocean anoxia, or it could be a result of particularly effective Fe (oxyhydr)oxide particulate shuttling.
The shuttling model is based on the experimental finding that lighter Mo isotopes become preferentially adsorbed to Fe (oxyhydr)oxides (Δ98Mo Fe (oxyhydr)oxides–seawater = −1.0 to −2.2 ‰; Goldberg et al. 2009) in the oxic zone. When Fe particulates encounter anoxic conditions, the isotopically light Mo in molybdate is desorbed and enhances bottom water or pore fluid Mo content compared to average seawater, and under sulfidic conditions (≥10 μM H2S) m) all Mo will precipitate as sulfide in the euxinic water column or in sediment pore water (Helz 2021).
Mercury
The metal-rich sulfide layer has on the order of 10–20 ppm Hg, the black shale on the order of 100 ppb Hg (Yin et al. 2017). The black shales and the metal-rich sulfide layer have a distinct mass-dependent fractionation signature of δ202HgNIST3133 in the range of −1 to −3 ‰ (Yin et al. 2017), with the metalliferous unit more negative, similar to the offset of −0.6 ‰ known from HgS formation (Foucher et al. 2013). More importantly, the metalliferous unit as well as the basal phosphorite and the barren black shale sequence all display mass-independent Hg isotope fractionation (Hg-MIF) with positive Δ199Hg values up to 0.24 ‰, a signal of photochemical processes, typical of seawater (Yin et al. 2017). However, Hg-MIF also characterizes all sedimentary rocks of any age, and the Hg-MIF signal could easily be inherited by basinal brines (Yin et al. 2016).
Selenium
The Ni-Mo-polymetallic sulfide ore bed at the Huangjiawan mine contains from 1069 to 2621 ppm Se, the surrounding carbonaceous black shale from 27 to 419 ppm Se (Lehmann et al. 2007; Wen and Carignan 2011), and the algal sapropelite (“stone coal”) has 20-50 ppm Se (Dai et al. 2018). Wen and Carignan (2011) reported a restricted range of δ82SeSRM3149 values (−1.6 to +2.4 ‰ with a mean of 0.6 ‰) in the whole sample set and δ82Se values close to 0 ‰ in the Huangjiawan ore sulfides. Rouxel et al. (2004) showed that δ82Se values of early-stage hydrothermal sulfides at the Lucky Strike hydrothermal field at the mid-Atlantic Ridge are consistently close to that of bulk Earth or mantle composition (δ82Se close to zero), similar to those measured in the Zunyi ore sulfides. Based on these findings Wen and Carignan (2011) suggested a dominantly hydrothermal origin where aqueous Se was probably transported as H2Se, along with H2S, and precipitated directly as selenides or in sulfides (Mo-S-C, pyrite, and millerite were identified as principal Se-bearing phases), leading to little or no isotopic fractionation between aqueous and solid species and subsequent Se remobilization by oxidizing fluids and then progressive reduction by organic-rich sediments.
However, Se is dominantly organic-bound in anoxic settings (Stüeken et al. 2015), and there is a distinct positive correlation between the abundance of Se and TOC in marine sediments (Pogge von Strandmann et al. 2015). The black shale of the Niutitang Formation has about 50 ppm Se at about 10 wt% TOC, indicating significant remineralization. A further loss of 90 % of organic matter by microbial activity would enhance the Se concentration tenfold, for example. Se would remain fixed quantitatively under sulfidic conditions, and would then also not fractionate, and essentially conserve the Se isotope composition of the decomposed plus remaining organic matter, with the Se isotope signal integrated over the initial sediment pile. The δ82Se value of about 0.0 ‰ is close to modern marine plankton of 0.42 ± 0.22 ‰, and close to modern seawater, because there is little or no Se isotope fractionation in organic materials (Mitchell et al. 2012). It is also within the global range of Neoproterozoic marine shales with −2 to +2 ‰ δ82Se (Pogge von Strandmann et al. 2015).
A tentative integrated view
The various metal isotope information discussed above can be seen as in accord with an ultimate seawater source of the metals in the highly metalliferous black shale unit, but cannot really exclude external hydrothermal metal input, such as hydrothermal exhalation of dense metalliferous brines into the euxinic water column. In particular, the intriguing negative Ni isotope signature of the polymetallic sulfide unit waits for a final interpretation, although particulate shuttling provides a possible explanation (Little et al. 2020). Other, more classical information has to be considered, such as geology. mineralogy and elemental and organic geochemistry. The strongest geochemical arguments are maybe the seawater-like patterns of REE and PGE, including Re and the radiogenic Os isotope composition, which were extensively discussed in Mao et al. (2002), Lehmann et al. (2007, 2016), and Xu et al. (2012, 2013). However, the close association of PGE with Fe-Ni sulfides also could suggest the involvement of mafic-related hydrothermal fluids in the formation of the sulfide-rich unit (Orberger et al. 2003, 2007; Li et al. 2013; Pašava et al. 2013). The degree of metal enrichment on the order of 107 is the intuitive problem of a seawater metal source, although the enrichment of the same metal spectrum is also observed in marine black shales (Vine and Tourtelot 1970; Holland 1979) but at an enrichment factor of 105–106 only. Also, there is 25–30 % pyrite in the metalliferous unit, which suggests that redox-controlled precipitation from a simple average seawater source is not sufficient, but involves redox cycling at and below the sediment-seawater interface, as observed in many modern and ancient environments (Berner and Raiswell 1983).
These questions, the tight spatial and temporal association of phosphate, barite and sulfide precipitation, PGE and REE patterns in sulfide and apatite, respectively, suggest the involvement of both seawater and hydrothermal sources (Murowchick et al. 1994; Pašava et al. 2008; Steiner et al. 2001; Zhu et al. 2020; Han et al. 2020; Fu et al. 2021), which was also proposed based on Ni isotope data (Pašava et al. 2019).
There is consensus that the Mo-Ni-rich metalliferous black shale unit formed in a marine sulfidic environment. Metal/TOC ratios in organic-rich sediments, particularly patterns of TOC/Mo covariation, are often used as proxies for benthic redox potential and paleohydrographic conditions and have been successfully applied to recent organic-rich sediments (Algeo and Lyons 2006). However, degradation of organic matter and thermal maturation, i.e., loss of organic carbon, will increase such ratios, and the extreme metal/TOC ratios in the sulfide-rich unit could relate to the massive loss of primary organic matter. In fact, the seawater model requires the loss of very much organic matter. The pioneering microanalytical S isotope work by Murowchick et al. (1994) on the peculiar pyrite nodules in the Zunyi district, with remnants of organic matter in apatite conservation, first indicated such an irreversible process. Murowchick et al. (1994) interpreted the cycling of S isotope composition at the μm-scale over a range of 48 ‰, and metal enrichment, as a result of massive microbial decomposition of organic debris in a sulfate-limited environment.
Pyrolysis experiments of organic-rich mudrocks on the example of the Early Jurassic Posidonia Shale have shown that thermal maturation does apparently not result in significant alteration of the isotopic compositions of Mo, Zn, and Cd in the rock residues, unlike Ni, which thus retains primary paleodepositional information. This is mainly due to the low solubilities of these elements, because experimental organic fluids have a lighter isotopic Zn composition by about 0.4–0.5 ‰ δ66Zn, while the δ98Mo composition of the organic fluid remains identical to the bulk rock (Dickson et al. 2020).
The high biological productivity at the margin of the Early Cambrian Yangtze Platform could be seen as a response to increased nutrient supply to the surface waters triggered by enhanced late Ediacaran oxidative weathering of the continents in the aftermath of the latest Neoproterozoic glaciations (Frei et al. 2021). Metal enrichment, phosphorites, and sapropelites reflect high bioproductivity and a stratified water column with oxic surface waters in the photic zone, and anoxic/euxinic waters below, similar to the Black Sea. However, different from the Black Sea, the paleohydrographic situation was such, that locally very low clastic input allowed the build-up of an essentially authigenic extremely metalliferous sulfide-rich unit. Such a situation seems to be rare. Also, the peculiar texture of the sulfide-rich marker unit with a high amount of partly sulfidized bioclastic components (rip-up clasts of organic matter/algal mats, indicating at least short-distance transport) and flattened or brecciated sulfide and phosphate nodules suggests a low sedimentation rate or even erosion and winnowing in the sense of a subaquatic hardground (Kříbek et al. 2007).
The closest analogue of the Early Cambrian metal-rich black shale unit is the mid-Devonian Nick prospect, Yukon, Canada (Hulbert et al. 1992; Goodfellow et al. 2010) and other occurrences along the 1000-km-long N-trending Selwyn basin of the Devonian continental margin of Ancestral North America (Fraser et al. 2018; Peter et al. 2018; Gadd et al. 2019, 2020). There are three stratigraphically different hyper-enriched shale units within a few m of the stratigraphic column (Gadd et al. 2019). The Nick prospect has a metal enrichment spectrum, and extreme Re and Os enrichment, similar to the South China situation, and recently gave a Re-Os isochron age of 391 ± 5 Ma with an initial 187Os/188Os of 0.3 ± 0.2 (Gadd et al. 2020). The black shale host rock, with much lower Re and Os, plots on the regression line, i.e., is coeval with the Re-Os rich unit. The relatively low initial 187Os/188Os ratio corresponds to the composition of the global mid-Devonian oceans, with a then apparently much lower global amount of continental weathering than in the early Cambrian. Therefore, particularly intense oxidative weathering seems not to be a prerequisite for the formation of highly metalliferous black shale. With respect to the metal enrichment in the mid-Devonian Nick prospect, the question remains if the strong Ni-Zn enrichment could not result from hydrothermal input as suggested by Hulbert et al. (1992), Orberger et al. (2003, 2005), and Pašava et al. (2019), although recent Mo and Tl isotope studies favor a seawater model (Crawford et al. 2021).
The global picture of the formation of highly metalliferous black shale units was described by Johnson et al. (2017) as a consequence of a feedback loop of an increase in oxidative erosion, which triggers increased supply of P and nutrient trace elements to the oceans, with a rise in bioproductivity, and finally leads to anoxic to euxinic bottom waters. However, in this general context of maximum organic-matter production and drawdown of redox-sensitive trace elements to the seafloor, it is those short-lived episodes where maximum organic matter is destroyed and sulfate-reducing microbes produce sulfide for efficient metal fixation, either in the water column, or at the water-sediment interface, or inside the uppermost sediment with pore water redox cycling. Key is efficient anaerobic carbon destruction, which may operate via sulfate reduction, i.e. 2CH2O + SO42- → H2S + 2HCO3-, or methanogenesis, i.e. 2CH2O → CH4 + CO2 (“CH2O” as a summary formula for biomass). The more detailed reaction of microbially-driven sulfate reduction, closer to the real composition of particulate organic matter and derived from studies on the Black Sea (Hiscock and Millero 2006), is (CH2O)130(NH3)16(H3PO4) + 65SO42- → 130HCO3- + 65H2S + 16NH3 + H3PO4.
This reaction then also explains the authigenic phosphate component in the polymetallic sulfide unit and calcite formation. The release of biogenic silica, not considered in this equation, depends on the relative abundance of siliceous phytoplankton. The observed ratio of Si(OH)4/H2S in the deep water of the Black Sea is 0.41 ± 0.01 (Hiscock and Millero 2006). The release of H2S and HCO3- increases total alkalinity to pH >8 in the Black Sea. High alkalinity can also explain the occasionally positive Eu anomaly in the REE pattern of some samples from the sulfide-rich marker unit (see Sverjensky 1984) which has been interpreted as a signal of hydrothermal fluids by some previous workers.
High biological productivity in the photic zone must be compensated by organic-matter remineralization and high activity of sulfate-reducing microbes. Otherwise, sapropelite will build up, as observed as a stratigraphically lateral equivalent with 10s of m thick combustible shale (Coveney et al. 1994). The industrially mined “stone coal” (ash content >50 wt%; inferred resource: 55 Gt; Dai et al. 2018) was previously widely used for domestic cooking and heating in South China and its elevated As and Se contents led to severe health problems. The bulk metal contents are about 100–200 ppm As, 20−50 ppm Se, 1000–2000 ppm V, 0.5–1 ppm Hg, ~200 ppm Ni, ~200 ppm Mo, 100–500 ppm Zn (Luo 2011; Dai et al. 2018).
Conclusions
The isotope data suggest a situation of paleoceanic conditions comparable to modern analogues such as the Black Sea, with active biochemical cycling induced by phototrophic microorganisms above the redoxcline, and euxinic conditions below. However, strong metal enrichment requires special conditions. These are as follows: (1) very low depositional rate to prevent dilution by clastic deposition; (2) large fluid flux in an upflow zone which allows renewal of seawater with or without hydrothermal input; (3) sulfate-reducing microbes to establish sulfidic conditions, where Mo, As, Ni and Zn can be precipitated, which all are also enriched in average seawater, particularly molybdenum (10 ppb Mo, 1.2 ppb As, 0.48 ppb Ni, 0.35 ppb Zn; Nozaki 1997); (4) Intense microbiological activity is required for advanced degradation of organic matter above, at or below the seafloor, where methanogens (or other microorganisms) could develop the specific negative Ni signature of the sediments. Negative Ni isotope composition could possibly also derive from the shuttling of Fe-Mn (oxyhyrd)oxides in the water column.
In stratigraphically equivalent, lateral non-sulfidic but anoxic environments, with again low depositional rate, V could become enriched from average seawater because V, similar to Mo, has a relatively high abundance (2 ppb V; Nozaki 1997). Under anoxic conditions in bottom waters and pore waters, dissolved vanadium is reduced to particle-reactive V4+ and is incorporated into the authigenic clay fraction (Piper 1994), well documented for the Lower Permian Phosphoria Formation or the Upper Carboniferous Mecca Quarry Shale in the western USA, for example (Peacor et al. 2000).
Metal enrichment to ore grade is possible in those parts of the basins where the clastic depositional rate is at a minimum and carbon loss is at a maximum. The hydrographic situation must be in a delicate balance between high oxic seawater renewal rate in the upper part of the water column, and stagnant anoxic conditions below, which prevent element dispersal, plus upwelling nutrient-rich deep water. Such a situation would apply to a rift environment of a passive continental margin, with a not only redox-stratified but also density-stratified water column, similar to the modern Black Sea. Note, however, that rifts in combination with basinal brines can have a variety of sub-seafloor fluid systems such as cold seeps and low-T hydrothermal vents, which may tap and leach unexposed mafic-ultramafic rocks in the basement. Basinal brines could also simply leach the thick Neoproterozoic limestone/shale sequence (Denying and Doushantuo formations) on the Yangtze Platform which includes metalliferous black shales. In summary, there is some room for doubt for the simple seawater model and a more diverse situation with vertical redox cycling and fluid venting at the seafloor may be more appropriate in a complex world.
References
Ackerman L, Pašava J, Šípková A, Martínková E, Haluzová E, Rodovská Z, Chrastný V (2019) Copper, zinc, chromium and osmium isotopic compositions of the Teplá-Barrandian unit black shales and implications for the composition and oxygenation of the Neoproterozoic-Cambrian ocean. Chem Geol 521:59–75
Algeo TJ, Lyons TW (2006) Mo-total organic carbon covariation in modern anoxic marine environments: implications for analysis of paleoredox and paleohydrographic conditions. Paleoceanography 21(PA1016):1–23
Archer C, Vance D, Milne A, Lohan MC (2020) The oceanic biogeochemistry of nickel and its isotopes: new data from the South Atlantic and the Southern Ocean biogeochemical divide. Earth Planet Sci Lett 535:116118
Barling J, Arnold GL, Anbar AD (2001) Natural mass-dependent variation in the isotopic composition of molybdenum. Earth Planet Sci Lett 193:447–457
Belkin HE, Luo K (2008) Late-stage sulfides and sulfarsenides in Lower Cambrian black shale (stone coal) from the Huangjiawan mine, Guizhou Province, People’s Republic of China. Mineral Petrol 92:321–340
Berner RA, Raiswell R (1983) Burial of organic carbon and pyrite sulfur in sediments over Phanerozoic time: a new theory. Geochim Cosmochim Acta 47:855–862
Borrok DM, Nimick DA, Wanty RB, Ridley WI (2008) Isotopic variations of dissolved copper and zinc in stream waters affected by historical mining. Geochim Cosmochim Acta 72:329–344
Bryan AL, Dickson AJ, Dowdall F, Homoky WB, Procelli D, Henderson GM (2021) Controls on the cadmium isotope composition of modern marine sediments. Earth Planet Sci Lett 565:116946
Cameron V, Vance D, Archer C, House CH (2009) A biomarker based on the stable isotopes of nickel. PNAS 106:10944–10948
Chapman JB, Mason TFD, Weiss DJ, Coles BJ, Wilkinson JJ (2006) Chemical separation and isotopic variations of Cu and Zn from five geological reference materials. Geostand Geoanalyt Res 30:5–16
Conway TM, John SG (2014) The biogeochemical cycling of zinc and zinc isotopes in the North Atlantic Ocean. Glob Biogeochem Cycles 28:1111–1128
Coveney RM Jr, Chen NS (1991) Ni-Mo-PGE-Au-rich ores in Chinese black shales and speculations on possible analogues in the United States. Miner Deposita 26:83–88
Coveney RM Jr, Grauch RI, Murowchick JB (1994) Metals, phosphate and stone coal in the Proterozoic and Cambrian of China: the geologic setting of precious metal-bearing Ni-Mo ore beds. Soc Econ Geol Newsl 18:6–11
Crawford I, Layton-Matthews D, Peter JM, Hadd MG, Voinot A, Leybourne MI, Pufahl P (2021) Application of molybdenum and thallium isotopes as indicators of paleoredox conditions and genesis of hyper-enriched black shale deposits, Peel river, Yukon, Canada. Can Mineral 59:1085–1110
Dai SF, Zheng X, Wang XB, Finkelman RB, Jiang YF, Ren DY, Yan XY, Zhou YP (2018) Stone coal in China: a review. Int Geol Rev 60:736–753
Decrée S, Pašava J, Baele JM, Mercadier J, Rösel D, Frimmel H (2021) In-situ trace element and Sr isotope signature of apatite: a new key to unravelling the genesis of polymetallic mineralisation in black shales of the Early Cambrian Niutitang Formation, Southern China. Ore Geol Rev under review
Dickson AJ, Idiz E, Porcelli D, van den Boorn SHJM (2020) The influence of thermal maturity on the stable isotope compositions and concentrations of molybdenum, zinc and cadmium in organic-rich marine mudrocks. Geochim Cosmochim Acta 287:205–220
Dong J, Zhang SH, Jiang GQ, Zhao QL, Li HY, Shi XY, Liu JL (2008) Early diagenetic growth of carbonate concretions in the upper Doushantuo Formation in South China and their significance for the assessment of hydrocarbon source rock. Sci China Ser D-Earth Sci 51:1330–1339
Emsbo P, Hofstra AH, Johnson CA, Koenig A, Grauch R, Zhang XC, Hu RZ, Su WC, Pi DH (2005) Lower Cambrian metallogenesis of south China: interplay between diverse basinal hydrothermal fluids and marine chemistry. In: Mao JW, Bierlein FP, eds, Mineral Deposit Research: Meeting the Global Challenge. Proceedings of the Eigth Biennial SGA Meeting Beijing, China, 18-21 August 2005, Vol 1, 115–118
Erickson BE, Helz GR (2000) Molybdenum (VI) speciation in sulfidic water: Stability and lability of thiomolybdates. Geochim Cosmochim Acta 64:1149–1158
Fan HF, Zhang HJ, Xiao CY, Pasava J, Han T, Zhou T, Wen HJ (2020) Large Zn isotope variations in the Ni\\Mo polymetallic sulfide layer in the lower Cambrian, South China. Gondwana Res 85:224–236
Foucher D, Hintelmann H, Al TA, MacQuarrie KT (2013) Mercury isotope fractionation in waters and sediments of the Murray Brook mine watershed (New Brunswick, Canada): tracing mercury contamination and transformation. Chem Geol 336:87–95
Fraser TA, Crawford I, Gadd MG, Henderson K, Layton-Matthews D, Melchin M, Peter JM, Sack PJ, Sperling E, Strauss J (2018) An overview of shale studies in Yukon during the 2017 field season. In: Yukon Exploration and Geology 2017, K.E. MacFarlane (ed.), Yukon Geol Surv, p. 37–45
Frei R, Lehmann B, Xu LG, Frederiksen JA (2020) Surface water oxygenation and bioproductivity – a link provided by combined chromium and cadmium isotopes in Early Cambrian metalliferous black shales (Nanhua Basin, South China). Chem Geol 552:119785
Frei R, Xu LG, Frederiksen JA, Lehmann B (2021) Signals of combined chromium–cadmium isotopes in basin waters of the Early Cambrian – results from the Maoshi and Zhijin sections, Yangtze Platform, South China. Chem Geol 563:120061
Fu Y, Yang Z, Li C, Xia P (2021) Enrichment of platinum group elements in Lower Cambrian polymetallic black shale, SE Yangtze Block, China. Front Earth Sci 9:651948
Fujii T, Moynier F, Pons M-L, Albarède F (2011) The origin of Zn isotope fractionation in sulphides. Geochim Cosmochim Acta 75:7632–7643
Fujii T, Moynier F, Blichert-Toft J, Albarède F (2014) Density functional theory estimation of isotope fractionation of Fe, Ni, Cu and Zn among species relevant to geochemical and biological environments. Geochim Cosmochim Acta 140:553–576
Gadd MG, Peter JM, Jackson SE, Yang ZP, Petts D (2019) Platinum, Pd, Mo, Au and Re deportment in hyper-enriched black shale Ni-Zn-Mo-PGE mineralization, Peel River, Yukon, Canada. Ore Geol Rev 107:600–614
Gadd MG, Peter JM, Hnatyshin D, Creaser R, Gouwy S, Fraser T (2020) A Middle Devonian basin-scale precious metal enrichment event across northern Yukon (Canada). Geology 48:242–246
Gall L, Williams HM, Halliday AN, Kerr AC (2017) Nickel isotopic composition of the mantle. Geochim Cosmochim Acta 199:196–209
Gao JB, Yang RD, Chen J, Zheng LL, Cheng W, Wei HR (2017) Multiple proxies indicating methane seepage as the origin of Devonian large barite deposit in Zhenning-Ziyun, Guizhou, SW China. Ore Geol Rev 80:18–26
Goldberg T, Mazumdar A, Strauss H, Shields G (2006) Insights from stable S and O isotopes into biogeochemical processes and genesis of Lower Cambrian barite–pyrite concretions of South China. Org Geochem 37:1278–1288
Goldberg T, Strauss H, Guo QJ, Liu CQ (2007) Reconstructing marine redox conditions for the early Cambrian Yangtze Platform: evidence from biogenic sulphur and organic carbon isotopes. Palaeogeogr Palaeoclimat Palaeoecol 254:175–193
Goldberg T, Archer C, Cance D, Poulton SW (2009) Mo isotope fractionation during adsorption to Fe (oxyhydr)oxides. Geochim Cosmochim Acta 73:6502–6516
Golden Share Mining Corp (2016) Golden share announces the strategic partnership agreement with Northwest Mining & Exploration Group. May 24, 2016. https://www.juniorminingnetwork.com/. Accessed 6 Feb 2022
Goodfellow WD, Geldsetzer H, Gregoire C, Orchard M, Cordey F (2010) Geochemistry and origin of geographically extensive Ni(Mo,Zn,U)-PGE sulphide deposits hosted in Devonian black shales, Yukon [abs.]: Geological Association of Canada, Cordilleran Section, TGI-3 Workshop: Public Geoscience in Support of Base Metal Exploration, March 22, 2010, Vancouver, Programme and Abstracts, p. 15–18
Granitto M, Giles SA, Kelley KD (2017) Global Geochemical Database for Critical Metals in Black Shales: U.S. Geological Survey data release, https://doi.org/10.5066/F71G0K7X.
Gueguen B, Rouxel O, Ponzevera E, Bekker A, Fouquet Y (2013) Nickel isotope variations in terrestrial silicate rocks and geological reference materials measured by MC-ICP-MS. Geostand Geoanal Res 37:297–317
Gueguen B, Rouxel O, Rouget ML, Bollinger C, Ponzevera E, Germain Y, Fouquet Y (2016) Comparative geochemistry of four ferromanganese crusts from the Pacific Ocean and significance for the use of Ni isotopes as paleoceanographic tracers. Geochim Cosmochim Acta 189:214–235
Gueguen B, Rouxel O, Fouquet Y (2021) Nickel isotopes and rare earth elements systematics in marine hydrogenetic and hydrothermal ferromanganese deposits. Chem Geol 560:119999
Guinoiseau D, Gélabert A, Moureau J, Louvat P, Benedetti MF (2016) Zn isotope fractionation during sorption onto kaolinite. Environ Sci Technol 50:1844–1852
Guo MY, Strauss H, Liu CQ, Goldberg T, Zhu MY, Pi DH, Heubeck C, Vernhet E, Yang XL, Fu PQ (2007) Carbon isotopic evolution of the terminal Neoproterozoic and early Cambrian: evidence from the Yangtze Platform, South China. Palaeogeogr Palaeoclimat Palaeoecol 254:140–157
Han SC, Hu K, Cao J, Pan JY, Xia F, Wu WF (2015) Origin of early Cambrian black-shale-hosted barite deposits in South China: mineralogical and geochemical studies. J Asian Earth Sci 195:79–94
Han T, Fan HF, Wen HJ, Mo B, Murowchick JB, Lu ZT, Algeo TJ (2020) Petrography and sulfur isotopic compositions of SEDEX ores in the early Cambrian Nanhua Basin, South China. Precambrian Res 345:105757
Helz GR (2021) Dissolved molybdenum asymptotes in sulfidic waters. Geochem Perspect Lett 19:23–26
Hohl SV, Jiang SY, Wei HZ, Pi DH, Liu Q, Viehmann S, Galer SJG (2019) Cd isotopes trace periodic (bio)geochemical metal cycling at the verge of the Cambrian animal evolution. Geochim Cosmochim Acta 263:195–214
Holland HD (1979) Metals in black shales - a reassessment. Econ Geol 74:1676–1679
Horan MF, Morgan JW, Grauch RI, Coveney RM Jr, Murowchick JB, Hulbert LJ (1994) Rhenium and osmium isotopes in black shales and Ni-Mo-PGE-rich sulfide layers, Yukon Territory, Canada, and Hunan and Guizhou provinces, China. Geochim Cosmochim Acta 58:257–265
Hsü KJH, Chen HH (1999) Geologic Atlas of China. Elsevier, Amsterdam
Huang J, Liu SA, Gao Y, Xiao Y, Chen S (2016) Copper and zinc isotope systematics of altered oceanic crust at IODP Site 1256 in the eastern equatorial Pacific. J Geophys Res Solid Earth 121:7086–7100
Huang S, Wang W (2019) Origin of the Fanjingshan mafic-ultramafic rocks, western Jiangnan Orogen, South China: implications for PGE fractionation and mineralisation. J Earth Sci 30:258–271
Hulbert LJ, Gregoire DC, Paktunc D, Carne RC (1992) Sedimentary nickel, zinc, and platinum-group-element mineralization in Devonian black shales at the Nick property, Yukon, Canada: A new deposit type. Explor Mining Geol 1:39–62
Ikehata K, Notsu K, Hirata T (2011) Copper isotope characteristics of copper-rich minerals from Besshi-type volcanogenic massive sulfide deposits, Japan, determined using a femtosecond LA-MC-ICP-MS. Econ Geol 106:307–316
Jia ZB, Hou DJ, Sun DQ, Jiang YH, Zhang ZM, Hong M (2018) Geochemical characteristics of source rocks in the Lower Cambrian Niutitang Formation in Guizhou Province, China. J Nat Gas Geosci 3:263–272
Jiang SY, Yang JH, Ling HF, Chen YQ, Feng HZ, Zhao KD, Ni P (2007) Extreme enrichment of polymetallic Ni-Mo-PGE-Au in Lower Cambrian black shales of South China: An Os isotope and PGE geochemical investigation. Palaeogeogr Palaeoclimat Palaeoecol 254:217–228
Jiang G, Shi X, Zhang S, Wang Y, Xiao S (2011) Stratigraphy and paleogeography of the Ediacaran Doushantuo Formation (ca. 635–551Ma) in South China. Gondwana Res 19:831–849
Jochum KP, Nohl U, Herwig K, Lammel E, Stoll B, Hofmann AW (2005) GeoReM: A new geochemical database for reference materials and isotopic standards. Geostand Geoanalyt Res 29: 333–338. http://georem.mpch-mainz.gwdg.de/. Accessed Jan 2021
John SG, Geis RW, Saito MA, Boyle EA (2007) Zinc isotope fractionation during high affinity and low-affinity zinc transport by the marine diatom Thalassiosira oceanica. Limnol Oceanogr 52:2710–2714
John S, Rouxel O, Craddock P, Engwall A, Boyle E (2008) Zinc stable isotopes in seafloor hydrothermal vent fluids and chimneys. Earth Planet Sci Lett 269:17–28
John G, Helgoe J, Townsend E (2018) Biogeochemical cycling of Zn and Cd and their stable isotopes in the Eastern Tropical South Pacific. Mar Chem 201:256–262
Johnson SC, Large RR, Coveney RM, Kelley KD, Slack JF, Steadman JA, Gregory DD, Sack PJ, Meffre S (2017) Secular distribution of highly metalliferous black shales corresponds with peaks in past atmosphere oxygenation. Miner Deposita 52:791–798
Jouvin D, Louvat P, Juillot F, Maréchal CN, Benedetti MF (2009) Zinc isotopic fractionation: why organic matters. Environ Sci Technol 43:5747–5754
Kao LS, Peacor DR, Coveney RM, Zhao GM, Dungey KE, Curtis MD, Penner-Hahn JE (2001) A C/MoS2 mixed-layer phase (MoSC) occurring in metalliferous black shales from southern China, and new data on jordisite. Am Mineral 86:852–861
Kelley KD, Wilkinson JJ, Chapman JP, Crowther HL, Weiss DJ (2009) Zinc isotopes in sphalerite from base metal deposits in the Red Dog district, northern Alaska. Econ Geol 104:767–773
Ketris MP, Yudovich YE (2009) Estimations of Clarkes for carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int J Coal Geol 78:135–148
Klaver M, Ionov DA, Takazawa E, Elliott T (2020) The non-chondritic Ni isotope composition of Earth’s mantle. Geochim Cosmochim Acta 268:405–421
Kříbek B, Sýkorová I, Pašava J, Machovič V (2007) Organic geochemistry and petrology of barren and Mo-Ni-PGE mineralized marine black shales of the Lower Cambrian Niutitang Formation (South China). Int J Coal Geol 72:240–256
Kurzweil F, Drost K, Pašava J, Wille M, Taubald H, Schoeckle D, Schoenberg R (2015) Coupled sulfur, iron and molybdenum isotope data from black shales of the Teplá-Barrandian unit argue against deep ocean oxygenation during the Ediacaran. Geochim Cosmochim Acta 171:121–142
Lacan F, Francois R, Ji YC, Sherrell RM (2006) Cadmium isotopic composition in the ocean. Geochim Cosmochim Acta 70:5104–5118
Lehmann B, Mao JW, Li SR, Zhang GD, Zeng MG (2003) Re-Os dating of polymetallic Ni-Mo-PGE-Au mineralization in lower Cambrian black shales of south China and its geological significance—a reply. Econ Geol 98:663–665
Lehmann B, Nägler TF, Holland HD, Wille M, Mao JW, Pan JY, Ma DS, Dulski P (2007) Highly metalliferous carbonaceous shale and Early Cambrian seawater. Geology 35:403–406
Lehmann B, Frei R, Xu LG, Mao JW (2016) Early Cambrian black shale-hosted Mo-Ni and V mineralization on the rifted margin of the Yangtze Platform, China: reconnaissance chromium isotope data and a refined metallogenic model. Econ Geol 111:89–103
Li X, Han T, Zhu X, Li Y, Zheng Y, Shen H (2013) A mineralogical application of micro-PIXE technique: the Ni-Mo-PGE polymetallic layer of black shales in Zunyi region, South China. Nucl Inst Methods Phys Res B 308:1–5
Li XQ, Zhang JZ, Wang Y, Guo M, Wang Z, Wang FY (2018) Accumulation condition and favorable area evaluation of shale gas from the Niutitang Formation in northern Guizhou, South China. J Nat Gas Geosci 3:1–10
Lin T, Bao SJ, Zhang JC, Zhou Z, Yuan K, Li B, Yang SY (2016) 2016, Shale gas accumulation conditions and gas-bearing characteristics of the Lower Cambrian Niutitang Formation in Well Changye-1 in northwestern Hunan Province. Pet Res 2:205–212
Little SH, Vance D, Walker-Brown C, Landing WM (2014) The oceanic mass balance of copper and zinc isotopes, investigated by analysis of their inputs, and outputs to ferromanganese oxide sediments. Geochim Cosmochim Acta 125:673–693
Little SH, Vance D, McManus J, Severmann S, Lyons TW (2017) Copper isotope signatures in modern marine sediments. Geochim Cosmochim Acta 312:253–273
Little SH, Archer C, McManus J, Najorka J, Wegorzewaki AV, Vance D (2020) Towards balancing the oceanic Ni budget. Earth Planet Sci Letts 547:116461
Lott DA, Coveney RM Jr, Murowchick JB, Grauch RI (1999) Sedimentary exhalative nickel-molybdenum ores in South China. Econ Geol 94:1051–1066
Luo KL (2011) Arsenic and fluorine contents and distribution patterns of Early Paleozoic stonelike coal in the Daba Fold Zone and Yangtze Plate, China. Energy Fuels 25:4479–4487
Lv YW, Liu SA, Zhu JM, Li SG (2016) Copper and zinc isotope fractionation during deposition and weathering of highly metalliferous black shales in central China. Chem Geol 445:24–35
Makishima A, Nakamura E (2013) Low-blank chemistry for Zn stable isotope ratio determination using extraction chromatographic resin and double spike-multiple collector-ICP-MS. J Anal Atom Spectrom 28:127–133
Mao J, Du A (2002) The 982 Ma Re-Os age of copper-nickel sulphide ores in the Baotan area, Guangxi and its geological significance. Sci China Ser D: Earth Sci 45(10):911–920
Mao JW, Lehmann B, Du AD, Zhang GD, Ma DS, Wang YT, Zeng MG, Kerrich R (2002) Re-Os dating of polymetallic Ni-Mo-PGE-Au mineralization in Lower Cambrian black shales of South China and its geologic significance. Econ Geol 97:1051–1061
Maréchal CN, Nicolas E, Douchet C, Albarède F (2000) Abundance of zinc isotopes as a marine biogeochemical tracer. Geochem Geophys Geosyst 1:5
Mathur R, Ruiz J, Titley S, Liermann L, Buss H, Brantley S (2005) Cu isotopic fractionation in the supergene environment with and without bacteria. Geochim Cosmochim Acta 69:5233–5246
Mathur R, Titley S, Barra F, Brantley S, Wilson M, Phillips A, Hart G (2009) Exploration potential of Cu isotope fractionation in porphyry copper deposits. J Geochem Explor 102:1–6
Mathur R, Jin L, Prush V, Paul J, Ebersole C, Fornadel A, Williams JZ, Brantley S (2012) Cu isotopes and concentrations during weathering of black shale of the Marcellus Formation, Huntingdon County, Pennsylvania (USA). Chem Geol 304–305:175–184
McFadden KA, Huang J, Chu XL, Jiang GQ, Kaufman AJ, Zhou CM, Yuan XL, Xiao SH (2008) Pulsed oxidation and biological evolution in the Ediacaran Doushantuo Formation. PNAS 105:3197–3202
Mitchell K, Mason PRD, van Cappellen P, Johnson TM, Gill BC, Owens JD, Diaz-Ellery J, Ingall D, Reichart GJ, Lyons TW (2012) Selenium as paleo-oceanographic proxy: a first assessment. Geochim Cosmochim Acta 89:302–317
Moynier F, Vance D, Fujii T, Savage P (2017) The isotope geochemistry of zinc and copper. Rev Miner Geochem 82:543–600
Murowchick JB, Coveney RM Jr, Grauch RI (1994) Cyclic variations of sulfur isotopes in Cambrian stratabound Ni-Mo-(PGE-Au) ores of southern China. Geochim Cosmochim Acta 58:1813–1823
Nägler T, Anbar AD, Archer C, Goldberg T, Gordon GW, Greber ND, Siebert C, Sohrin Y, Vance D (2013) Proposal for an international molybdenum isotope measurement standard and data representation. Geostand Geoanalyt Res 38https://doi.org/10.1111/j.1751-908X.2013.00275.x
Nakagawa Y, Takano S, Firdaus ML, Norisuye K, HirataT Vance D, Sohrin Y (2012) The molybdenum isotopic composition of the modern ocean. Geochem J 46:131–141
Novák M, Šípková A, Chrastný V, Štěpánová M, Voldřichová P, Veselovský F, Přechová E, Bláha V, Čuřík J, Farkaš J, Erbanová L, Bohdálková L, Pašava J, Míková J, Komárek A, Krachler M (2016) Cu-Zn isotope constraints on the provenance of air pollution in Central Europe: using soluble and insoluble particles in snow and rime. Environ Pollut 218:1135–1146
Nozaki Y (1997) A fresh look at element distribution in the North Pacific. EOS (Transact, Am Geophys Union) 78:221. https://doi.org/10.1029/97EO00148
Och LM, Shields-Zhou GA, Poulton SW, Manning C, Thirlwall MF, Li D, Chen X, Ling HF, Osborn T, Cremonese L (2013) Redox changes in Early Cambrian black shales at Xiaotan section, Yunnan province, South China. Precambrian Res 225:166–189
Orberger B, Pašava J, Gallien JP, Daudin L, Pinti D (2003) Biogenic and abiogenic hydrothermal sulfides: controls of rare metal distribution in black shales (Yukon Territories, Canada). J Geochem Explor 78–79:559–563
Orberger B, Gallien JP, Pinti D, FialinM Daudin L, Gröcke DR, Pašava J (2005) Nitrogen and carbon partitioning in diagenetic and hydrothermal minerals from Paleozoic black shales (Selwyn Basin, Yukon Territories, Canada). Chem Geol 218:249–264
Orberger B, Vymazalova A, Wagner C, Fialin M, Gallien JP, Wirth R, Pašava J, Montagnac G (2007) Biogenic origin of intergrown Mo-sulphide- and carbonaceous matter in Lower Cambrian black shales (Zunyi Formation, southern China). Chem Geol 238:213–231
Pagès A, Barnes S, Schmid S, Coveney R, Schwark L, Liu W, Grice K, Fan H, Wen H (2018) Geochemical investigation of the lower Cambrian mineralised black shales of South China and the late Devonian Nick deposit, Canada. Ore Geol Rev 94:396–413
Pašava J, Kříbek B, Vymazalová A, Sýkorová I, Žák K, Orberger B (2008) Multiple sources of metals of mineralization in Lower Cambrian black shales of South China: Evidence from geochemical and petrographic study. Resour Geol 58:25–42
Pašava J, Zaccarini F, Aiglsperger T, Vymazalová A (2013) Platinum-group elements (PGE) and their principal carriers in metal-rich black shales: an overview with a new data from Mo–Ni–PGE black shales (Zunyi region, Guizhou Province, south China). J Geosci 58:213–220
Pašava J, Chrastný V, Loukola-Ruskeeniemi K, Šebek O (2019) Nickel isotopic variation in black shales from Bohemia, China, Canada, and Finland: a reconnaissance study. Mineral Deposita 54:719–742
Peacor DR, Coveney RM Jr, Zhao GM (2000) Authigenic illite and organic matter: the principal hosts pf vanadium in the Mecca Quarry Shale at Velpen, Indiana. Clays Clay Miner 48:311–316
Peter JM, Bocking N, Gadd MG, Layton-Matthews D, Johnson N (2018) Textural and mineralogical characterization of a Ni-Zn-rich black shale occurrence at the Akie property, Kechika Trough, northern British Columbia, and comparison with examples from Yukon; in Targeted Geoscience Initiative: 2017 Report of Activities, Vol 1, (ed) N. Rogers; Geol Surv Canada, Open File 8358, p. 207–216
Pichat S, Douchet C, Albarède F (2003) Zinc isotope variations in deep-sea carbonates from the eastern equatorial Pacific over the last 175 ka. Earth Planet Sci Lett 210:167–178
Piper DZ (1994) Seawater as the source of minor elements in black shales, phosphorites, and other sedimentary rocks. Chem Geol 114:95–114
Piper DZ, Calvert SE (2009) A marine biogeochemical perspective on black shale deposition. Earth Sci Rev 95:63–96
Pogge von Strandmann PAE, Stüeken Elliott T, Poulton SW, Dehler CM, Canfield DE, Catling DC (2015) Selenium isotope evidence for progressive oxidation of the Neoproterozoic biosphere. Nat Commun 6:10157
Pokrovsky OS, Viers J, Emnova EE, Kompantseva EI, Freydier R (2008) Copper isotope fractionation during its interaction with soil and aquatic microorganisms and metal oxy(hyd)oxides: possible structural control. Geochim Cosmochim Acta 72:1742–1757
Ratié G, Jouvin D, Garnier J, Rouxel O, Miska S, Guimarães E, Cruz Vieira L, Sivry Y, Zelanod I, Montarges-Pelletier E, Thil F, Quantin C (2015) Nickel isotope fractionation during tropical weathering of ultramafic rocks. Chem Geol 402:68–76
Ratié G, Garnier J, Calmels D, Vantelon D, Guimarães E, Monvoisin G, Nouet J, Ponzevera E, Quantin C (2018) Nickel distribution and isotopic fractionation in a Brazilian lateritic regolith: coupling Ni isotopes and Ni K-edge XANES. Geochim Cosmochim Acta 230:137–154
Reinhard CT, Planavsky NJ, Wang XL, Fischer WW, Johnson TM, Lyons TW (2014) The isotopic composition of authigenic chromium in anoxic marine sediments: a case study from the Cariaco basin. Earth Planet Sci Lett 407:9–18
Revels BN, Rickli J, Moura CAV, Vance D (2021) Nickel and its isotopes in the Amazon Basin: the impact of the weathering regime and delivery to the oceans. Geochim Cosmochim Acta 293:344–364
Rouxel O, Fouquet Y, Ludden JN (2004) Subsurface processes at the Lucky strike hydrothermal field, Mid-Atlantic ridge: evidence from sulfur, selenium, and iron isotopes. Geochim Cosmochim Acta 68:2295–2311
Scheller S, Goenrich M, Boecher R, Thauer RK, Jaun B (2010) The key nickel enzyme of methanogenesis catalyses the anaerobic oxidation of methane. Nature 465:606–608
Scherer P, Lippert H, Wolff G (1983) Composition of the major elements and trace elements of 10 methanogenic bacteria determined by inductively coupled plasma emission spectrometry. Biol Trace Elem Res 5:149–163
Schoenberg R, Zink S, Staubwasser M, von Blanckenburg F (2008) The stable Cr isotope inventory of solid Earth reservoirs determined by double spike MC-ICP-MS. Chem Geol 249:294–306
Scholz F, McManus J, Sommer S (2013) The manganese and iron shuttle in a modern euxinic basin and implications for molybdenum cycling at euxinic ocean margins. Chem Geol 355:56–68
Scotese CR, 2003, Paleomap Project. http://www.scotese.com. Accessed 6 Feb 2022
Sieber M, Conway TM, de Souza GF, Obata H, Takano S, Sohrin Y, Vance D (2019) Physical and biogeochemical controls on the distribution of dissolved cadmium and its isotopes in the Southwest Pacific Ocean. Chem Geol 511:494–509
Sorensen JV, Gueguen B, Stewart BD, Peña J, Rouxel O, Toner BM (2020) Large nickel isotope fractionation caused by surface complexation reactions with hexagonal birnessite. Chem Geol 537:119481
Sossi PA, Halverson GP, Nebel O, Eggins SM (2015) Combined separation of Cu, Fe and Zn from rock matrices and improved analytical protocols for stable isotope determination. Geostand Geoanal Res 39:129–149
Steiner M, Wallis E, Erdtmann BD, Zhao YL, Yang RD (2001) Submarine-hydrothermal exhalative ore layers in black shales from South China and associated fossils: Insights into a Lower Cambrian facies and bio-evolution. Palaeogeogr Palaeoclimat Palaeoecol 169:165–191
Stüeken EE, Buick R, Bekker A, Catling D, Foriel J, Guy BM, Kah LC, Machel HG, Montanez P, Poulton W (2015) The evolution of the global selenium cycle: secular trends in Se isotopes and abundances. Geochim Cosmochim Acta 162:109–125
Sverjensky DA (1984) Europium redox equilibria in aqueous solution. Earth Planet Sci Lett 67:70–78
Sweere TC, Dickson AJ, Jenkyns C, Procelli D, Ruhl M, Murphy MJ, Sander E, van den Boorn HJM, Eldrett JS, Henderson GM (2020) Controls on the Cd-isotope composition of Upper Cretaceous (Cenomanian–Turonian) organic-rich mudrocks from south Texas (Eagle Ford Group). Geochim Cosmochim Acta 287:251–262
Takano S, Tanimizu M, Hirata T, Sohrin Y (2014) Isotopic constraints on biogeochemical cycling of copper in the ocean. Nat Commun 5:5663
Tatzel M, von Blanckenburg F, Oelzel M, Bouchez J, Hippler D (2015) Late Neoproterozoic seawater oxygenation by siliceous sponges. Nat Commun 8:621
Taylor SR, McLennan SM (1985) The continental crust: its composition and evolution. Blackwell, 312 p
Teng FZ, Dauphas N, Watkins JM (2017) Non-traditional stable isotopes: Retrospective and prospective. Rev Miner Geochem 82:1–26
Vance D, Archer C, Bermin J, Perkins J, Statham PJ, Lohan MC, Ellwood MJ, Mills RA (2008) The copper isotope geochemistry of rivers and the oceans. Earth Planet Sci Lett 274:204–213
Vance D, Little SH, Archer C, Cameron V, Andersen MB, Rijkenberg MJA, Lyons TW (2016) The oceanic budgets of nickel and zinc isotopes: the importance of sulfidic environments as illustrated by the Black Sea. Philos Trans A Math Phys Eng Sci 374(2081):20150294
Vernhet E, Heubeck C, Zhu MY, Zhang JM (2006) Large-scale slope instability at the southern margin of the Ediacaran Yangtze platform (Hunan province, central China). Precambrian Res 148:32–44
Vine JD, Tourtelot EB (1970) Geochemistry of black shale deposits— a summary report. Econ Geol 65:253–272
Voldřichová P, Chrastný V, Šípková A, Farkaš J, Novák M, Štěpánová M, Krachler M, Veselovský F, Bláha V, Přechová E, Komárek A, Bohdálková L, Čuřík J, Míková J, Erbanová L, Pacherová P (2014) Zinc isotope systematics in snow and ice accretions in Central European mountains. Chem Geol 388:130–141
Wallis E (2007) Die Klima- und Umweltgeschichte der südchinesischen Yangtze Plattform im Neoproterozoikum und frühen Kambrium: hydrothermal aktive, Dichte-stratifizierte Epikontinentalbecken, der Schlüssel zum Verständnis der „Kambrischen Explosion“? PhD thesis, Techn Univ Berlin, 227 p
Wang HZ, Chu XC, Liu BP, Hou HF, Ma LF, eds, 1985, Atlas of the Paleogeography of China. Beijing, Cartographic Publishing House, 143 p
Wang ZC, Li GZ (1991) Barite and witherite deposits in Lower Cambrian shales of South China: stratigraphic distribution and geochemical characterization. Econ Geol 86:354–363
Wang PF, Jiang ZX, Han B, Lv P, Jin C, Zhang K (2018) Reservoir characteristics and controlling factor of shale gas in Lower Cambrian Niutitang Formation, South China. Pet Res 3:210–220
Wang XC, Li XH, Li WX, Li ZX (2007) Ca. 825 Ma komatiitic basalts in South China: first evidence for >1500°C mantle melts by a Rodinian mantle plume. Geology 35:1103–1106
Wen HJ, Carignan J (2011) Selenium isotopes trace the source and redox processes in the black shale-hosted Se-rich deposits in China. Geochim Cosmochim Acta 75:1411–1427
Wen HJ, Zhu CW, Zhang YX, Cloquet C, Fan HF, Fu SH (2016) Zn/Cd ratios and cadmium isotope evidence for the classification of lead-zinc deposits. Sci Rep 6:25273
Wilkinson JJ, Weiss DJ, Mason TFD, Coles BJ (2005) Zinc isotope variation in hydrothermal systems: preliminary evidence from the Irish Midlands ore field. Econ Geol 100:583–590
Wille M, Nägler TF, Lehmann B, Schröder S, Kramers JD (2008) Hydrogen sulphide release to surface waters at the Precambrian/Cambrian boundary. Nature 453:767–769
Wu T, Yang RD, Gao JB, Li J (2021) Age of the lower Cambrian vanadium deposit, East Guizhou, South China: evidences from age of tuff and carbon isotope analysis along the Bagong section. Open Geosci 13:999–1012
Xu LG (2011) Early Cambrian black shale and associated polymetallic Ni-Mo-PGE-Au mineralization, South China. PhD thesis, Techn Univ Clausthal, 369 p
Xu LG, Mao JW (2021) Trace element and C-S-Fe geochemistry of Early Cambrian black shales and associated polymetallic Ni-Mo sulfide and vanadium mineralization, South China: Implications for paleoceanic redox variation. Ore Geol Rev 135:104210
Xu LG, Lehmann B, Mao JW, Qu WJ, Du AD (2011) Re-Os age of polymetallic Ni-Mo-PGE-Au mineralization in Early Cambrian black shales of South China—a reassessment. Econ Geol 106:511–522
Xu LG, Lehmann B, Mao JW, Nägler TF, Neubert N, Böttcher ME, Escher P (2012) Mo isotope and trace element patterns of Lower Cambrian black shales in South China: multi-proxy constraints on the paleoenvironment. Chem Geol 318–319:45–59
Xu LG, Lehmann B, Mao JW (2013) Seawater contribution to polymetallic Ni-Mo-PGE-Au mineralization in Early Cambrian black shales of South China: evidence from Mo isotope, PGE, trace element, and REE geochemistry. Ore Geol Rev 52:66–84
Xu LG, Lehmann B, Mao JW, Zheng W, Ye HS, Li HY (2016) Strontium, sulfur, carbon, and oxygen isotope geochemistry of the Early Cambrian strata-bound barite and witherite deposits of the Qinling-Daba region, northern margin of the Yangtze Craton, China. Econ Geol 111:695–718
Yao WH, Li ZX, Li WX, Li XH, Yang JH (2014) From Rodinia to Gondwanaland: a tale of detrital zircon provenance analyses from the southern Nanhua Basin, South China. Am J Sci 314:278–313
Ye YH, Luo C, Liu SG, Xiao C, Ran B, Sun W, Yang D, Luba J, Zeng XL (2017) 2017, Characteristics of black shale reservoirs and controlling factors of gas adsorption in the Lower Cambrian Niutitang Formation in the southern Yangtze basin margin, China. Energy Fuels 31:6876–6894
Yin L, Li J, Tian H, Long XP (2018) Rhenium-osmium and molybdenum isotope systematics of black shales from the Lower Cambrian Niutitang Formation, SW China: Evidence of a well oxygenated ocean at ca. 520 Ma. Chem Geol 499:26–42
Yin RS, Feng XB, Hurley JP, Krabbenhoft DP, Lepak RF, Hu RZ, Zhang QA, Li ZG, Bi XW (2016) Mercury isotopes as proxies to identify sources and environmental impacts of mercury in sphalerites. Sci Rep 6:18686
Yin RS, Xu LG, Lehmann B, Lepak RF, Hurley JP, Mao JW, Feng XB, Hu RH (2017) Anomalous mercury enrichment in Early Cambrian black shales of South China: mercury isotopes indicate a seawater source. Chem Geol 467:159–167
Zhang JP, Fan TL, Algeo TJ, Li YF (2016) Paleo-marine environments of the Early Cambrian Yangtze Platform. Palaeogeogr Palaeoclimat Palaeoecol 443:66–79
Zhang K, Peng J, Wang X, Jiang ZX, Song Y, Jiang L, Jiang S, Xue ZX, Wen M, Li XH, Liu XX, Huang YZ, Wang PF, Shan CA, Liu TL, Xie XL (2020) Effect of organic maturity on shale gas genesis and pores development: a case study on marine shale in the upper Yangtze region, South China. Open Geosci 12:1617–1629
Zhao G, Cawood PA (2012) Precambrian geology of China. Precambrian Res 222–223:13–54
Zhao ZQ, Shen B, Zhu JM, Lang XG, Wu GL, Tan D, Pei HX, Huang TZ, Ning M, Ma HR (2021) Active methanogenesis during the melting of Marinoan snowball Earth. Nat Commun 12:955
Zheng JP, Griffin WL, O’Reilly SY, Zhang M, Pearson N, Pan YM (2006) Widespread Archean basement beneath the Yangtze craton. Geology 34:417–420
Zhou JX, Huang ZL, Zhou MF, Zhu XK, Muchez P (2014) Zinc, sulfur and lead isotopic variations in carbonate-hosted Pb–Zn sulfide deposits, southwest China. Ore Geol Rev 58:41–54
Zhou Y, Zhong H, Li C, Ripley EM, Zhu WG, Bai ZJ, Li C (2017) Geochronological and geochemical constraints on sulphide mineralisation in the Qingmingshan mafic intrusion in the western part of the Proterozoic Jiangnan orogenic belt along the southern margin of the Yangtze craton. Ore Geol Rev 90:618–633
Zhu CW, Wang J, Zhang JW, Chen XC, Fan HF, Zhang YX, Yant T, Wen HJ (2020) Isotope geochemistry of Zn, Pb and S in the Ediacaran strata hosted Zn-Pb deposits in Southwest China. Ore Geol Rev 117:103274
Žák K, Pašava J, Vymazalová A, Kříbek B, Li C, Hailin D, Zeng M (2003) Ni-Mo-PGE rich black shales of South China: preliminary results from the isotope study of related barite and carbonates. In Eliopoulos DG, ed, Mineral Exploration and Sustainable Development, Proceedings of the 7th Biennial SGA Meeting; 24–28 August 2003, Athens, Greece, Vol 2, 861–864
Acknowledgements
This is a contribution to the Strategic Research Plan of the Czech Geological Survey (DKRVO 2018-2022) and to the Czech Science Foundation (Grantova Agentura České Republiky) project 17-15700S. LX thanks the National Natural Science Foundation of China (No. 41972072). We thank Editor Georges Beaudoin and Associate Editor Fernando Tornos for manuscript handling and critical reading, and the reviewers Ryan Mathur and Sophie Decrée for helpful comments which much improved an earlier manuscript version.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Editorial handling: F. Tornos/G. Beaudoin
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lehmann, B., Pašava, J., Šebek, O. et al. Early Cambrian highly metalliferous black shale in South China: Cu and Zn isotopes and a short review of other non-traditional stable isotopes. Miner Deposita 57, 1167–1187 (2022). https://doi.org/10.1007/s00126-022-01097-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00126-022-01097-0