Comparative Study of the Structural Properties, Color, Bioactive Compounds Content and Antioxidant Capacity of Aerated Gelatin Gels Enriched with Cryoconcentrated Blueberry Juice during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CBJ Preparation
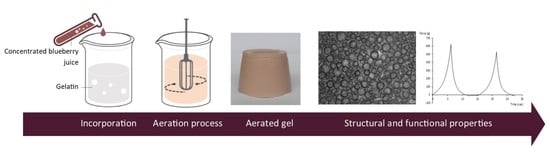
2.3. CBJ Incorporation into Aerated Gelatin Gels
2.4. Apparent Viscosity
2.5. Density and Gas Hold-Up Measurements
2.6. Texture Profile Analysis (TPA)
2.7. Bubble Size Quantification by Image Analysis
2.8. Color Analysis
2.9. Determination of Total Bioactive Compounds Content (TBCC)
2.10. Determination of Antioxidant Activity (AA)
2.11. Analysis of Individual Phenolic Compounds (IPC) by High-Performance Liquid Chromatography (HPLC)
2.12. Determination of Recovery Percentage (RP)
2.13. Statistical Analysis
3. Results and Discussion
3.1. Apparent Viscosity, Density and Gas Hold-Up Measurements
3.2. Texture Profile Analysis (TPA)
3.3. Bubble Size Quantification
3.4. Color Measurement
3.5. Determination of the Total Bioactive Compounds Content and Antioxidant Activity
3.6. Analysis of Individual Phenolic Compounds (IPC) by HPLC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perez-Puyana, V.; Jiménez-Rosado, M.; Rubio-Valle, J.F.; Guerrero, A.; Romero, A. Gelatin vs. collagen-based sponges: Evaluation of concentration, additives and biocomposites. J. Polym. Res. 2019, 26, 190. [Google Scholar] [CrossRef]
- Lee, D.H.; Tamura, A.; Arisaka, Y.; Seo, J.H.; Yui, N. Mechanically reinforced gelatin hydrogels by introducing slidable supramolecular cross-linkers. Polymers 2019, 11, 1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalik, R.; Wandzik, I.A. Mini-review on chitosan-based hydrogels with potential for sustainable agricultural applications. Polymers 2020, 12, 2425. [Google Scholar] [CrossRef] [PubMed]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Fabrication and characterization of hydrogels based on gelatinised collagen with potential application in tissue engineering. Polymers 2020, 12, 1146. [Google Scholar] [CrossRef]
- Zúñiga, R.N.; Aguilera, J.M. Aerated food gels: Fabrication and potential applications. Trends Food Sci. Technol. 2008, 19, 176–187. [Google Scholar] [CrossRef]
- de Oliveira, J.P.; Bruni, G.P.; Fonseca, L.M.; da Silva, F.T.; da Rocha, J.C.; da Rosa Zavareze, E. Characterization of aerogels as bioactive delivery vehicles produced through the valorization of yerba-mate (Illex paraguariensis). Food Hydrocoll. 2020, 107, 105931. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Gondek, E.; Kamińska-Dwórznicka, A.; Samborska, K.; Wiktor, A.; Królikowski, K. A complex approach to assessing properties of aerated agar-fructose gels: Application of acoustic emission technique. Food Hydrocoll. 2019, 91, 66–75. [Google Scholar] [CrossRef]
- Mondal, A.; Niranjan, K. The role of bubbles in the development of food structure. In Handbook of Food Structure Development; Spyropoulos, F., Lazidis, A., Norton, I., Eds.; Royal Society of Chemistry Publisher: Cambridge, UK, 2019; pp. 93–114. [Google Scholar]
- Orrego, M.; Troncoso, E.; Zúñiga, R.N. Aerated whey protein gels as new food matrices: Effect of thermal treatment over microstructure and textural properties. J. Food Eng. 2015, 163, 37–44. [Google Scholar] [CrossRef]
- Zheng, Q.; Tian, Y.; Ye, F.; Zhou, Y.; Zhao, G. Fabrication and application of starch-based aerogel: Technical strategies. Trends Food Sci. Technol. 2020, 99, 608–620. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Takhar, P.; Petzold, G. Increasing the separation of block cryoconcentration through a novel centrifugal filter-based method. Sep. Sci. Technol. 2019, 54, 786–794. [Google Scholar] [CrossRef]
- Petzold, G.; Orellana-Palma, P.; Moreno, J.; Junod, J.; Bugueño, G. Freeze concentration as a technique to protect valuable heat labile components of foods. In Innovative Processing Technologies for Foods with Bioactive Compounds; Moreno, J., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 183–194. [Google Scholar]
- Pardo, J.M.; Moya-Albor, E.; Ortega-Ibarra, G.; Brieva, J. Freezing front velocity estimation using image processing techniques. Measurement 2020, 151, 107085. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Lazo-Mercado, V.; Gianelli, M.P.; Hernández, E.; Zúñiga, R.N.; Petzold, G. Influence of cryoconcentration on quality attributes of apple juice (Malus Domestica cv. Red Fuji). Appl. Sci. 2020, 10, 959. [Google Scholar] [CrossRef] [Green Version]
- Kowalczewski, P.Ł.; Olejnik, A.; Białas, W.; Kubiak, P.; Siger, A.; Nowicki, M.; Lewandowicz, G. Effect of thermal processing on antioxidant activity and cytotoxicity of waste potato juice. Open Life Sci. 2019, 14, 150–157. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Petzold, G.; Andana, I.; Torres, N.; Cuevas, C. Retention of ascorbic acid and solid concentration via centrifugal freeze concentration of orange juice. J. Food Qual. 2017, 2017, 5214909. [Google Scholar] [CrossRef] [Green Version]
- Orellana-Palma, P.; Petzold, G.; Torres, N.; Moreno, J.; Aguilera, M. Elaboration of orange juice concentrate by vacuum-assisted block freeze concentration. J. Food Process. Preserv. 2018, 42, 13438. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; González, Y.; Petzold, G. Improvement of centrifugal cryoconcentration by ice recovery applied to orange juice. Chem. Eng. Technol. 2019, 42, 925–931. [Google Scholar] [CrossRef]
- Adorno, W.T.; Rezzadori, K.; Arend, G.D.; Chaves, V.C.; Reginatto, F.H.; di Luccio, M.; Petrus, J.C. Enhancement of phenolic compounds content and antioxidant activity of strawberry (Fragaria × ananassa) juice by block freeze concentration technology. Int. J. Food Sci. Technol. 2017, 52, 781–787. [Google Scholar] [CrossRef]
- Safiei, N.Z.; Ngadi, N.; Johari, A.; Zakaria, Z.Y.; Jusoh, M. Grape juice concentration by progressive freeze concentrator sequence system. J. Food Process. Preserv. 2017, 41, e12910. [Google Scholar] [CrossRef]
- Petzold, G.; Moreno, J.; Lastra, P.; Rojas, K.; Orellana, P. Block freeze concentration assisted by centrifugation applied to blueberry and pineapple juices. Innov. Food Sci. Emerg. Technol. 2015, 30, 192–197. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Petzold, G.; Guerra-Valle, M.; Astudillo-Lagos, M. Impact of block cryoconcentration on polyphenol retention in blueberry juice. Food Biosci. 2017, 20, 149–158. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Petzold, G.; Pierre, L.; Pensaben, J.M. Protection of polyphenols in blueberry juice by vacuum-assisted block freeze concentration. Food Chem. Toxicol. 2017, 109, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, A.A.; Zardo, D.M.; Alberti, A.; Bortolini, D.G.; Benvenutti, L.; Demiate, I.M.; Nogueira, A. Effect of cryoconcentration process on phenolic compounds and antioxidant activity in apple juice. J. Sci. Food Agric. 2019, 99, 2786–2792. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Qin, F.G.; Yuan, J.; Huang, S.; Jiang, R.; Shao, Y. Concentration of apple juice with an intelligent freeze concentrator. J. Food Eng. 2019, 256, 61–72. [Google Scholar] [CrossRef]
- Qin, F.G.; Ding, Z.; Peng, K.; Yuan, J.; Huang, S.; Jiang, R.; Shao, Y. Freeze concentration of apple juice followed by centrifugation of ice packed bed. J. Food Eng. 2020, 291, 110270. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Zúñiga, R.N.; Takhar, P.S.; Gianelli, M.P.; Petzold, G. Effects of centrifugal block freeze crystallization on quality properties in pineapple juice. Chem. Eng. Technol. 2020, 2, 355–364. [Google Scholar] [CrossRef]
- Henao-Ardila, A.; Quintanilla-Carvajal, M.X.; Moreno, F.L. Combination of freeze concentration and spray drying for the production of feijoa (Acca sellowiana b.) pulp powder. Powder Technol. 2019, 344, 190–198. [Google Scholar] [CrossRef]
- Albergamo, A.; Costa, R.; Bartolomeo, G.; Rando, R.; Vadalá, R.; Nava, V.; Gervasi, T.; Toscano, G.; Ditta, F. Grape water: Reclaim and valorization of a by-product from the industrial cryoconcentration of grape (Vitis vinifera) must. J. Sci. Food Agric. 2020, 100, 2971–2981. [Google Scholar] [CrossRef]
- de Liz, G.R.; Verruck, S.; Machado-Canella, M.H.; Dantas, A.; Grechi-Garcia, S.; Maran, B.M.; Murakami, F.S.; Prudencio, E.S. Stability of bifidobacteria entrapped in goat’s whey freeze concentrate and inulin as wall materials and powder properties. Food Res. Int. 2020, 127, 108752. [Google Scholar] [CrossRef]
- da Silveira Lazzarotto, S.R.; Lazzarotto, M.; da Silveira, A.C.; Wendling, I.; Schnitzler, E. Corn starch incorporated with freeze-concentrated Ilex paraguariensis extracts: A potential nutraceutical product. J. Therm. Anal. Calorim. 2020, in press. [Google Scholar] [CrossRef]
- Guerra-Valle, M.E.; Moreno, J.; Lillo-Pérez, S.; Petzold, G.; Simpson, R.; Nuñez, H. Enrichment of apple slices with bioactive compounds from pomegranate cryoconcentrated juice as an osmodehydration agent. J. Food Qual. 2018, 2018, 7241981. [Google Scholar] [CrossRef]
- Jaster, H.; Arend, G.D.; Rezzadori, K.; Chaves, V.C.; Reginatto, F.H.; Petrus, J.C. Enhancement of antioxidant activity and physicochemical properties of yogurt enriched with concentrated strawberry pulp obtained by block freeze concentration. Food Res. Int. 2018, 104, 119–125. [Google Scholar] [CrossRef]
- Casas-Forero, N.; Orellana-Palma, P.; Petzold, G. Influence of block freeze concentration and evaporation on physicochemical properties, bioactive compounds and antioxidant activity in blueberry juice. Food Sci. Technol. 2020, in press. [Google Scholar] [CrossRef]
- Dai, H.; Li, X.; Du, J.; Ma, L.; Yu, Y.; Zhou, H.; Guo, T.; Zhang, Y. Effect of interaction between sorbitol and gelatin on gelatin properties and its mechanism under different citric acid concentrations. Food Hydrocoll. 2020, 101, 105557. [Google Scholar] [CrossRef]
- Moraes, I.C.; Carvalho, R.A.; Bittante, A.M.Q.; Solorza-Feria, J.; Sobral, P.J. Film forming solutions based on gelatin and poly(vinyl alcohol) blends: Thermal and rheological characterizations. J. Food Eng. 2009, 95, 588–596. [Google Scholar] [CrossRef]
- Cai, L.; Feng, J.; Regenstein, J.; Lv, Y.; Li, J. Confectionery gels: Effects of low calorie sweeteners on the rheological properties and microstructure of fish gelatin. Food Hydrocoll. 2017, 67, 157–165. [Google Scholar] [CrossRef]
- Petzold, G.; Orellana-Palma, P.; Moreno, J.; Cerda, E.; Parra, P. Vacuum-assisted block freeze concentration applied to wine. Innov. Food Sci. Emerg. Technol. 2016, 36, 330–335. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. CPFAC 2002, 6, I1.1.1–I1.1.8. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.; Wrolstad, R. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Vergara, C.; von Baer, D.; Zapata, M.; Hitschfeld, A.; Obando, L.; Mardones, C. Anthocyanin profiles in south Patagonian wild berries by HPLC-DAD-ESI-MS/MS. Food Res. Int. 2013, 51, 706–713. [Google Scholar] [CrossRef]
- Pineda-Vadillo, C.; Nau, F.; Guerin-Dubiard, C.; Jardin, J.; Lechevalier, V.; Sanz-Buenhombre, M.; Guadarrama, A.; Tóth, T.; Csavajda, É.; Hingyi, H.; et al. The food matrix affects the anthocyanin profile of fortified egg and dairy matrices during processing and in vitro digestion. Food Chem. 2017, 214, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Badii, F.; Howell, N.K. Fish gelatin: Structure, gelling properties and interaction with egg albumen proteins. Food Hydrocoll. 2006, 20, 630–640. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Sabzipour, F.; Taghizadeh, M.S.; Moosavi-Nasab, M. Physicochemical, rheological, and molecular characterization of colloidal gelatin produced from Common carp by-products using microwave and ultrasound-assisted extraction. J. Texture Stud. 2019, 50, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Mantelet, M.; Panouillé, M.; Boué, F.; Bosc, V.; Restagno, F.; Souchon, I.; Mathieu, V. Impact of sol-gel transition on the ultrasonic properties of complex model foods: Application to agar/gelatin gels and emulsion filled gels. Food Hydrocoll. 2019, 87, 506–518. [Google Scholar] [CrossRef]
- Lazidis, A.; de Almeida Parizotto, L.; Spyropoulos, F.; Norton, I.T. Microstructural design of aerated food systems by soft-solid materials. Food Hydrocoll. 2017, 78, 26–35. [Google Scholar] [CrossRef]
- O’Chiu, E.; Vardhanabhuti, B. Utilizing whey protein isolate and polysaccharide complexes to stabilize aerated dairy gels. J. Dairy Sci. 2017, 100, 3404–3412. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Benjakul, S.; Prodpran, T. Effect of chitooligosaccharide from squid pen on gel properties of sardine surimi gel and its stability during refrigerated storage. Int. J. Food Sci. Technol. 2019, 54, 2831–2838. [Google Scholar] [CrossRef]
- Suebsaen, K.; Suksatit, B.; Kanha, N.; Laokuldilok, T. Instrumental characterization of banana dessert gels for the elderly with dysphagia. Food Biosci. 2019, 32, 100477. [Google Scholar] [CrossRef]
- Ullah, I.; Hu, Y.; You, J.; Yin, T.; Xiong, S.; Din, Z.U.; Huang, Q.; Liu, R. Influence of okara dietary fiber with varying particle sizes on gelling properties, water state and microstructure of tofu gel. Food Hydrocoll. 2019, 89, 512–522. [Google Scholar] [CrossRef]
- Zúñiga, R.N.; Kulozik, U.; Aguilera, J.M. Ultrasonic generation of aerated gelatin gels stabilized by whey protein β-lactoglobulin. Food Hydrocoll. 2011, 25, 958–967. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Mieszkowska, A.; Olsiński, I.; Lenart, A. The effect of composition and aeration on selected physical and sensory properties of freeze-dried hydrocolloid gels. Food Hydrocoll. 2017, 67, 94–103. [Google Scholar] [CrossRef]
- Ellis, A.L.; Mills, T.B.; Norton, I.T.; Norton-Welch, A.B. The effect of sugars on agar fluid gels and the stabilisation of their foams. Food Hydrocoll. 2019, 87, 371–381. [Google Scholar] [CrossRef]
- Zúñiga, R.N.; Aguilera, J.M. Structure–fracture relationships in gas-filled gelatin gels. Food Hydrocoll. 2009, 23, 1351–1357. [Google Scholar] [CrossRef]
- Valenzuela, C.; Aguilera, J.M. Aerated apple leathers: Effect of microstructure on drying and mechanical properties. Dry. Technol. 2013, 31, 1951–1959. [Google Scholar] [CrossRef]
- Muhammad-Zahir, S.A.D.; Yahaya, O.K.M.; Omar, A.F. Correlating the natural color of tropical fruit juice with its pH. Color Res. Appl. 2020, in press. [Google Scholar] [CrossRef]
- Orellana-Palma, P.; Tobar-Bolaños, G.; Casas-Forero, N.; Zúñiga, R.N.; Petzold, G. Quality attributes of cryoconcentrated calafate (Berberis microphylla) juice during refrigerated storage. Foods 2020, 9, 1314. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, G.; Wang, W.; Yue, J.; Yue, P.; Gao, X. Anthocyanin profile, color and antioxidant activity of blueberry (Vaccinium ashei) juice as affected by thermal pretreatment. Int. J. Food Prop. 2019, 22, 1036–1046. [Google Scholar] [CrossRef] [Green Version]
- Rivero, R.; Archaina, D.; Sosa, N.; Leiva, G.; Baldi-Coronel, B.; Schebor, C. Development of healthy gummy jellies containing honey and propolis. J. Sci. Food Agric. 2020, 100, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Hanani, Z.N.; Yee, F.C.; Nor-Khaizura, M.A.R. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Kia, E.M.; Ghaderzadeh, S.; Langroodi, A.M.; Ghasempour, Z.; Ehsani, A. Red beet extract usage in gelatin/gellan based gummy candy formulation introducing Salix aegyptiaca distillate as a flavouring agent. J. Food Sci. Technol. 2020, 57, 3355–3362. [Google Scholar] [CrossRef]
- Pertuzatti, P.B.; Barcia, M.T.; Rodrigues, D.; da Cruz, P.N.; Hermosín-Gutiérrez, I.; Smith, R.; Godoy, H.T. Antioxidant activity of hydrophilic and lipophilic extracts of Brazilian blueberries. Food Chem. 2014, 164, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.; Xu, X.; Zhou, X.; Liu, Y.; Zhao, Z. Changes in phenolic profiles and antioxidant activity in rabbiteye blueberries during ripening. Int. J. Food Prop. 2019, 22, 320–329. [Google Scholar] [CrossRef] [Green Version]
- Putnik, P.; Kresoja, Ž.; Bosiljkov, T.; Jambrak, A.R.; Barba, F.J.; Lorenzo, J.M.; Roohinejad, S.; Granato, D.; Žuntar, I.; Kovačević, D.B. Comparing the effects of thermal and non-thermal technologies on pomegranate juice quality: A review. Food Chem. 2019, 279, 150–161. [Google Scholar] [CrossRef]
- Tarone, A.G.; Cazarin, C.B.B.; Junior, M.R.M. Anthocyanins: New techniques and challenges in microencapsulation. Food Res. Int. 2020, 133, 109092. [Google Scholar] [CrossRef]
- Correa, L.J.; Ruiz, R.Y.; Moreno, F.L. Effect of falling-film freeze concentration on bioactive compounds in aqueous coffee extract. J. Food Process. Eng. 2018, 41, e12606. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, M.; Yang, F.; Liu, J. Antioxidant activity of high purity blueberry anthocyanins and the effects on human intestinal microbiota. LWT Food Sci. Technol. 2020, 117, 108621. [Google Scholar] [CrossRef]
- Ochmian, I.; Błaszak, M.; Lachowicz, S.; Piwowarczyk, R. The impact of cultivation systems on the nutritional and phytochemical content, and microbiological contamination of highbush blueberry. Sci. Rep. 2020, 10, 16696. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Xing, K.; Zhang, X.X.; Wang, H.; Wang, Y.; Wang, F.; Li, J.M. Influence of freeze concentration technique on aromatic and phenolic compounds, color attributes, and sensory properties of Cabernet Sauvignon wine. Molecules 2017, 22, 899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.M.M.; de la Caba, K.; Prodpran, T.; Benjakul, S. Quality characteristics of fried fish crackers packaged in gelatin bags: Effect of squalene and storage time. Food Hydrocoll. 2020, 99, 105378. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Z. Effects of gelatin-polyphenol and gelatin–genipin cross-linking on the structure of gelatin hydrogels. Int. J. Food Prop. 2017, 20, S2822–S2832. [Google Scholar] [CrossRef]






| Sample | Ingredients (g) | ||
|---|---|---|---|
| Gelatin Gel | CBJ | Water | |
| M1 | 14 | 20 | 100 |
| M2 | 13 | 25 | 100 |
| M3 | 11 | 30 | 100 |
| M4 | 10 | 35 | 100 |
| M5 | 8 | 40 | 100 |
| M1 | M2 | M3 | M4 | M5 | |
|---|---|---|---|---|---|
| Viscosity (kPa·s) | 31.0 ± 1.8 e | 19.0 ± 1.1 d | 14.22 ± 1.2 c | 8.4 ± 0.8 ab | 7.1 ± 0.5 a |
| ρacg (kg/m3) | 511.3 ± 24.3 de | 490.5 ± 19.0 cd | 456.4 ± 17.9 c | 387.6 ± 29.9 ab | 349.0 ± 19.6 a |
| ε (%) | 49.1 ± 1.1 a | 52.1 ± 1.7 b | 57.1 ± 1.6 c | 64.2 ± 2.0 d | 67.1 ± 0.8 e |
| Day | M1 | M2 | M3 | M4 | M5 | |
|---|---|---|---|---|---|---|
| Hardness (N) | 1 | 33.44 ± 0.42 e,B | 25.62 ± 0.88 d,B | 20.08 ± 0.60 c,B | 13.63 ± 0.11 b,B | 10.51 ± 0.23 a,B |
| 7 | 32.25 ± 1.18 e | 24.50 ± 2.06 d | 19.44 ± 2.06 c | 13.05 ± 0.03 b | 10.09 ± 1.04 a | |
| 14 | 30.59 ± 0.48 e | 23.37 ± 0.97 d | 17.89 ± 1.22 c | 12.26 ± 0.23 b | 9.73 ± 0.19 a | |
| 21 | 28.16 ± 0.60 e | 22.56 ± 0.71 d | 16.25 ± 0.50 c | 11.43 ± 1.25 b | 8.33 ± 0.55 a | |
| 28 | 22.83 ± 1.57 e,A | 17.75 ± 1.31 d,A | 14.58 ± 0.44 c,A | 10.65 ± 0.12 b,A | 7.26 ± 0.13 a,A | |
| Springiness | 1 | 0.94 ± 0.00 d,B | 0.92 ± 0.01 b,c,B | 0.90 ± 0.01 a,b,B | 0.89 ± 0.02 a,b,B | 0.89 ± 0.01 a,B |
| 7 | 0.92 ± 0.04 c,d | 0.91 ± 0.01 b,c | 0.89 ± 0.02 a,b | 0.88 ± 0.02 a | 0.88 ± 0.02 a | |
| 14 | 0.90 ± 0.03 c,d | 0.90 ± 0.03 c,d | 0.88 ± 0.02 c | 0.84 ± 0.01 a,b | 0.82 ± 0.02 a | |
| 21 | 0.88 ± 0.02 d,e | 0.87 ± 0.02 d | 0.84 ± 0.00 c | 0.82 ± 0.00 b | 0.80 ± 0.01 a | |
| 28 | 0.88 ± 0.01 d,e,A | 0.86 ± 0.01 d,A | 0.82 ± 0.01 b,c,A | 0.81 ± 0.01 a,b,A | 0.78 ± 0.02 a,A | |
| Cohesiveness | 1 | 0.91 ± 0.02 d,e,B | 0.88 ± 0.04 c,d,B | 0.87 ± 0.02 c,B | 0.80 ± 0.03 a,b,B | 0.76 ± 0.02 a,B |
| 7 | 0.87 ± 0.02 d,e | 0.84 ± 0.02 c,d | 0.82 ± 0.03 c | 0.75 ± 0.03 a,b | 0.73 ± 0.03 a | |
| 14 | 0.84 ± 0.03 d,e | 0.83 ± 0.02 c,d | 0.80 ± 0.01 c | 0.74 ± 0.01 b | 0.71 ± 0.01 a | |
| 21 | 0.83 ± 0.03 d,e | 0.79 ± 0.03 c,d | 0.78 ± 0.02 c | 0.73 ± 0.02 a,b | 0.70 ± 0.02 a | |
| 28 | 0.81 ± 0.02 d,A | 0.76 ± 0.02 b,c,A | 0.74 ± 0.01 b,A | 0.73 ± 0.03 a,b,A | 0.70 ± 0.02 a,A | |
| Gumminess (N) | 1 | 30.53 ± 0.49 e,B | 22.60 ± 0.59 d,B | 17.46 ± 0.30 c,B | 10.84 ± 0.49 b,B | 7.98 ± 0.42 a,B |
| 7 | 28.15 ± 0.60 e | 19.74 ± 1.23 d | 16.05 ± 2.09 c | 9.56 ± 0.37 b | 7.42 ± 1.09 a | |
| 14 | 25.64 ± 1.02 e | 19.03 ± 1.21 d | 14.61 ± 0.82 c | 8.85 ± 0.13 b | 6.68 ± 0.20 a | |
| 21 | 23.31 ± 0.67 e | 17.31 ± 0.17 d | 12.60 ± 0.08 c | 8.45 ± 0.94 b | 5.83 ± 0.38 a | |
| 28 | 18.44 ± 1.34 e,A | 13.49 ± 1.08 d,A | 11.48 ± 0.29 c,A | 7.82 ± 0.36 b,A | 5.06 ± 0.22 a,A | |
| Chewiness (N) | 1 | 28.51 ± 0.51 e,B | 20.49 ± 1.04 d,B | 16.15 ± 0.35 c,B | 9.66 ± 0.78 b,B | 7.12 ± 0.23 a,B |
| 7 | 25.44 ± 1.20 e | 17.87 ± 1.18 d | 14.32 ± 2.80 c | 8.39 ± 0.27 b | 6.51 ± 0.84 a | |
| 14 | 22.45 ± 1.25 e | 17.17 ± 1.53 d | 12.79 ± 0.42 c | 7.42 ± 0.19 b | 5.74 ± 0.27 a | |
| 21 | 21.29 ± 0.68 e | 14.61 ± 0.77 d | 10.56 ± 0.05 c | 7.12 ± 0.78 b | 5.27 ± 0.40 a | |
| 28 | 16.21 ± 1.25 e,A | 12.08 ± 1.09 d,A | 9.46 ± 0.27 c,A | 7.06 ± 0.39 b,A | 4.35 ± 0.36 a,A |
| Day | M1 | M2 | M3 | M4 | M5 | |
|---|---|---|---|---|---|---|
| Anthocyanins | ||||||
| Delphinidin | 1 | 3.12 ± 0.04 b,c,B | 3.06 ± 0.08 a,b,B | 3.02 ± 0.04 a,B | 3.35 ± 0.06 d,B | 4.12 ± 0.01 e,B |
| 28 | 2.97 ± 0.03 A | 2.98 ± 0.02 A | 2.95 ± 0.02 A | 2.94 ± 0.01 a,A | 3.18 ± 0.05 A | |
| Cyanidin | 1 | 1.77 ± 0.01 a,B | 1.98 ± 0.05 b,B | 2.07 ± 0.03 c,B | 2.36 ± 0.01 d,B | 2.94 ± 0.08 e,B |
| 28 | 0.77 ± 0.00 c,A | 0.70 ± 0.01 b,A | 0.66 ± 0.01 a,A | 1.21 ± 0.03 d,A | 1.34 ± 0.00 e,A | |
| Malvidin | 1 | 4.88 ± 0.04 c,B | 4.58 ± 0.12 a,B | 4.63 ± 0.19 a,b,B | 4.86 ± 0.26 b,c,B | 5.45 ± 0.30 d,B |
| 28 | 4.59 ± 0.10 d,A | 4.41 ± 0.00 c,A | 4.28 ± 0.04 a,A | 4.40 ± 0.00 b,A | 4.60 ± 0.17 d,e,A | |
| Flavonoids | ||||||
| Epigallocatechin gallate | 1 | 8.17 ± 0.21 a,B | 11.01 ± 0.56 c,B | 13.64 ± 0.49 e,B | 12.24 ± 0.15 d,B | 9.18 ± 0.21 b,B |
| 28 | 7.89 ± 0.03 b,A | 7.93 ± 0.17 b,c,A | 10.77 ± 0.11 e,A | 9.87 ± 0.28 d,A | 7.68 ± 0.17 a,A | |
| Epicatechin | 1 | 3.34 ± 0.00 a,B | 3.84 ± 0.17 b,A | 4.44 ± 0.13 c,A | 5.59 ± 0.07 d,A | 6.61 ± 0.10 e,A |
| 28 | 1.05 ± 0.03 c,A | 0.83 ± 0.01 a,A | 1.14 ± 0.01 d,A | 0.84 ± 0.02 a,b,A | 1.24 ± 0.04 e,A | |
| Quercetin | 1 | 3.99 ± 0.11 a,b,A,B | 5.70 ± 0.00 e,A | 4.67 ± 0.12 d,B | 3.85 ± 0.09 a,B | 4.07 ± 0.09 b,c,B |
| 28 | 3.93 ± 0.01 e,A | 3.84 ± 0.00 d,A | 3.81 ± 0.02 c,A | 3.67 ± 0.07 b,A | 3.58 ± 0.00 a,A | |
| Myricetin | 1 | 2.96 ± 0.03 b,c,A,B | 3.10 ± 0.00 e,B | 2.81 ± 0.11 a,B | 3.05 ± 0.03 d,B | 2.92 ± 0.03 a,b,B |
| 28 | 2.93 ± 0.06 c,A | 3.01 ± 0.04 d,e,A | 2.63 ± 0.04 a,A | 3.00 ± 0.04 c,d,A | 2.64 ± 0.04 a,b,A | |
| Phenolic acids | ||||||
| Caffeic acid (CA) | 1 | 5.07 ± 0.18 a,A,B | 5.79 ± 0.07 b,B | 5.89 ± 0.02 c,B | 5.92 ± 0.07 b,c,d,B | 6.11 ± 0.04 e,B |
| 28 | 5.05 ± 0.02 a,A | 5.35 ± 0.13 b,A | 5.61 ± 0.04 c,A | 5.65 ± 0.03 c,d,A | 5.98 ± 0.01 e,A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas-Forero, N.; Orellana-Palma, P.; Petzold, G. Comparative Study of the Structural Properties, Color, Bioactive Compounds Content and Antioxidant Capacity of Aerated Gelatin Gels Enriched with Cryoconcentrated Blueberry Juice during Storage. Polymers 2020, 12, 2769. https://doi.org/10.3390/polym12122769
Casas-Forero N, Orellana-Palma P, Petzold G. Comparative Study of the Structural Properties, Color, Bioactive Compounds Content and Antioxidant Capacity of Aerated Gelatin Gels Enriched with Cryoconcentrated Blueberry Juice during Storage. Polymers. 2020; 12(12):2769. https://doi.org/10.3390/polym12122769
Chicago/Turabian StyleCasas-Forero, Nidia, Patricio Orellana-Palma, and Guillermo Petzold. 2020. "Comparative Study of the Structural Properties, Color, Bioactive Compounds Content and Antioxidant Capacity of Aerated Gelatin Gels Enriched with Cryoconcentrated Blueberry Juice during Storage" Polymers 12, no. 12: 2769. https://doi.org/10.3390/polym12122769